Introduction
Aspergillus species are common fungi found all over the world, with about 150–250 species identified. The ones that most often affect humans are Aspergillus fumigatus (AF), Aspergillus flavus, and Aspergillus niger.1 Allergic Bronchopulmonary Aspergillosis (ABPA) is a lung disease driven by the immune system's overreaction, primarily through an IgE-mediated type I hypersensitivity. However, type III (IgG-mediated) and type IV (cell-mediated) hypersensitivity reactions also play a role.2, 3 A genetic predisposition is thought to be a key factor in the development of ABPA. In people with a genetic tendency, inhaled Aspergillus fumigatus spores grow into hyphae, which release antigens that trigger an exaggerated immune response.1, 3 These antigens can impair the normal clearing of mucus and damage the airway lining, leading to an overactive immune response in the lungs. In addition to the immune reaction caused by these antigens, enzymes (proteases) from Aspergillus fumigatus can damage epithelial cells and weaken the airway’s protective barrier, further intensifying the immune response. These proteases also promote inflammation by stimulating cytokine production, like IL-8, and releasing growth factors, which can lead to tissue damage and bronchiectasis. Most ABPA cases are found in patients with bronchial asthma or cystic fibrosis. 4, 5 The diagnosis of ABPA is typically made by meeting criteria established by the International Society for Human and Animal Mycology (ISHAM). 6
Case Presentation
We report the case of a 24-year-old female student who had been experiencing symptoms for two and a half years before being diagnosed with Allergic Bronchopulmonary Aspergillosis (ABPA). Initially, she had occasional episodes of coughing up muddy, particulate matter once or twice a week, along with throat irritation. Over the next year, she developed left-sided nasal congestion and a runny nose, which was managed with anti-allergic medications. However, the frequency of her sputum production gradually increased. One month before she came to our hospital, she experienced two episodes of coughing up blood and had been coughing up brownish sputum. Concerned, she consulted a local doctor who recommended further tests and treatment. The patient did not smoke and had no exposure to biomass fuels but did have a history of seasonal allergies. Her father mentioned that she had recurrent shortness of breath as a child, and her mother had a history of asthma and was treated for tuberculosis based on radiological findings. The patient did not report any fever, weight loss, or loss of appetite.
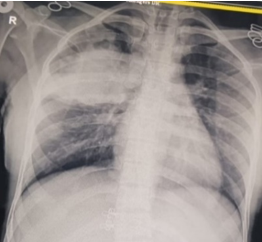
Figure 4
X-ray chest PA view suggestive of hydropneumothorax and after intercostal drainage insertion

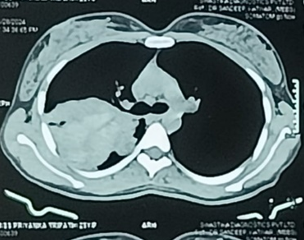
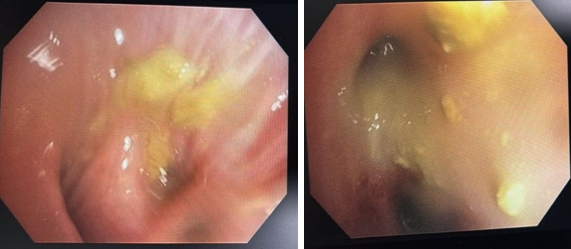
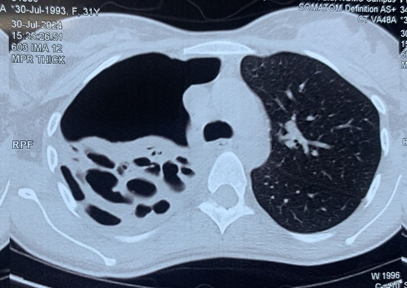
She was initially evaluated at another hospital, where tuberculosis or cancer was suspected. She was then referred to our center for further evaluation. On examination, she appeared well without any signs of respiratory distress, although she had mild pallor. There were no other significant findings on general examination, except for slightly decreased breath sounds on the right side. Initial chest X-ray findings (Figure 1) showed a radiopaque shadow in the right upper lung region. In contrast, subsequent CT imaging (Figure 2) revealed a dense, mass-like lesion suggestive of necrotizing tissue. Bronchoscopic examination (Figure 3) confirmed thick mucopurulent secretions. Further tests revealed an elevated Absolute Eosinophil Count of 116, serum total IgE levels over 2500 IU/ml, and significantly high levels of Aspergillus-specific IgE and IgG. Based on these findings, we confirmed the diagnosis of ABPA, and the patient was started on low-dose corticosteroid therapy (prednisolone 0.5 mg/kg/day) to control inflammation while minimizing infection risk. However, steroid therapy was paused after a bacterial superinfection with Klebsiella pneumoniae. A bronchoscopy revealed thick mucopurulent secretions from the right upper lobe due to suspicion of a high-attenuation mucus (HAM) plug. The bronchoalveolar lavage (BAL) fluid, which was yellow-green, indicated an infection, and KOH staining showed fungal elements with branched, septated hyphae. One week after starting treatment, the patient suddenly developed right-sided chest pain and shortness of breath following a night of coughing. On examination, there was a left-sided tracheal shift, reduced chest expansion, and no air entry on the right side. A chest X-ray (Figure 4) showed a right-sided hydropneumothorax. We immediately inserted an intercostal drain (Figure 4, Figure 5) and began conservative management. The pleural fluid analysis revealed foul-smelling pus, which tested negative for Mycobacterium tuberculosis but positive for Klebsiella pneumoniae (which was pan-sensitive). Due to the infection, we had to discontinue steroid treatment.
Further assessment revealed that the lung failed to re-expand and that there was a persistent air leak. A repeat scan showed a collapsed and destroyed lung with a bronchopleural fistula. We consulted with a thoracic surgeon, who recommended a right thoracotomy with bronchopleural fistula repair and possible decortication. However, due to the patient’s condition, immediate surgery was not advised, and she was scheduled for surgery after a period of nutritional buildup. The patient was discharged with antifungal treatment and was scheduled to restart steroids in 1–2 weeks.
Discussion
This case stands out due to its rare and unusual presentation of Allergic Bronchopulmonary Aspergillosis (ABPA), involving both pyothorax and a necrotizing mass lesion. According to the revised ISHAM-ABPA criteria (2024), diagnostic markers include serum total IgE levels ≥ 500 IU/mL, Aspergillus fumigatus-specific IgE ≥ 0.35 kUA/L, positive Aspergillus-specific IgG, eosinophilia ≥ 500 cells/µL, and characteristic radiological features such as central bronchiectasis, high-attenuation mucus, and fleeting pulmonary opacities.6 These criteria were fulfilled in our patient, with serum total IgE exceeding 2500 IU/mL, eosinophil counts above the diagnostic threshold, and imaging findings indicating necrotizing mass and pyopneumothorax, which, though not classic features, suggest severe disease progression. While ABPA is typically associated with conditions like asthma and cystic fibrosis, the simultaneous occurrence of pyothorax and a necrotizing mass lesion as initial complications is highly uncommon. It has not been documented before, making this case a valuable addition to the medical literature. ABPA is an immune system disorder triggered by a hypersensitive reaction to Aspergillus fumigatus. The immune response in ABPA involves type I, III, and IV hypersensitivity reactions, which lead to inflammation in the airways, mucus plugging, and bronchiectasis.7 In this patient, the necrotizing mass lesion likely resulted from severe bronchial obstruction caused by mucus plugs, combined with an inflammatory response and tissue death (necrosis). Many times patients are misdiagnosed as having pulmonary tuberculosis and given ATT.8, 9 Although ABPA can cause secondary pneumothorax in advanced stages of the disease—characterized by lung fibrosis and bronchiectasis 10 —our patient was not at such an advanced stage. Previous reports, such as one by Judson et al., describe cases where patients with ABPA developed secondary spontaneous pneumothorax and bronchopleural fistula during mucus removal from bronchiectatic airways. It was suggested that these complications might arise due to the formation of sub-pleural cystic spaces caused by air trapping behind thick mucus plugs. Another report by Das et al. described a patient who presented with spontaneous pneumothorax at the time of ABPA diagnosis and later developed lobar collapse on the same side. 7
Severe Asthma with Fungal Sensitization (SAFS) shares several immunologic features with ABPA but differs in key diagnostic, clinical, and management aspects. Unlike ABPA, SAFS does not present with central bronchiectasis on chest imaging, and its diagnostic criteria emphasize lower IgE levels without requiring evidence of Aspergillus fumigatus-specific IgE. Additionally, SAFS typically occurs in patients with severe asthma without radiological findings of bronchiectasis or mucous plugging. In our patient, the presence of central bronchiectasis, highly elevated total, and specific IgE, and compatible imaging findings confirmed ABPA over SAFS, guiding the treatment approach toward antifungal and corticosteroid therapy. Early differentiation between these conditions is critical, as treatment protocols and disease progression differ significantly. 11, 12
In our case, the development of pyothorax could be attributed to the rupture of a necrotizing mass into the pleural space, which is a rare but possible complication given the aggressive inflammation seen in ABPA. The presence of Klebsiella pneumoniae in the pleural fluid indicates a secondary bacterial infection, which worsened the pleural effusion and led to pyothorax.
This case highlights the importance of clinicians recognizing atypical presentations of ABPA, especially when faced with mass-like lesions in the lungs. Early diagnosis and treatment are essential to prevent serious complications, such as pyothorax and bronchopleural fistula, as occurred in this patient.
Treatment of ABPA typically includes corticosteroids to reduce inflammation and antifungal medications to control Aspergillus colonization. 6 However, in cases with a concurrent bacterial infection, as seen here, corticosteroids must be managed carefully to avoid worsening the infection. In this patient, steroid treatment was halted due to the presence of Klebsiella pneumoniae, and appropriate antibiotic therapy was initiated.
Previous studies, such as those by Gefter and colleagues, have observed cavitation in some cases of ABPA, often due to obstructive atelectasis from mucus plugs leading to post-obstructive abscesses, or tissue necrosis within large alveolar infiltrates. ABPA may also be complicated by secondary lung abscesses caused by pyogenic organisms, including Streptococcus pneumoniae. 13 However, there is limited documentation of pyothorax and necrotizing mass lesions as initial presentations of ABPA. This case not only aligns with isolated reports of cavitary lesions in ABPA but also introduces the progression to pyothorax as a novel finding.
Conclusion
This case highlights the complexity and variability in the presentation of ABPA. Clinicians should consider ABPA in the differential diagnosis of patients with mass-like pulmonary lesions, particularly in those with a history of asthma or atopy. Comprehensive evaluation using clinical, immunological, and radiological data is essential for accurate diagnosis. Prompt recognition and tailored management of complications such as pyothorax, can significantly impact patient outcomes.
Future research should focus on understanding the mechanisms underlying such rare presentations of ABPA and developing guidelines for the management of complex cases involving bacterial superinfections and structural lung changes.