- Visibility 258 Views
- Downloads 113 Downloads
- Permissions
- DOI 10.18231/j.ijirm.2024.020
-
CrossMark
- Citation
Conceptual therapeutics for small cell lung cancer
Abstract
Small-cell lung cancer (SCLC) is 15% of all lung cancers and is definitely of the most aggressive tumors, with relative poor prognosis and with limited drugs. 90% of patients of SCLC are regular smokers providing the link that tobacco carcinogens are responsible for the initiation. The value of female smokers (79.9) is more than that of male smokers (20.3). The 5 year survival rate has been reported to be less than 7%. The cornerstone of treatment for any stage of SCLC is etoposide-platinum based chemotherapy; in limited stage (LS) along with concomitant radiotherapy to thorax and mediastinum. Novel drugs used in treatment of SCLC are: Atezolizumab. Everolimus, Durvalumab, Iplimumab, Tarlatamab which target PD-L1, a protein related to PD-1 that is found on some tumor cells and immune cells.The combination of thoracic RT with chemotherapy results in improved survival compared to chemotherapy alone.The role of surgery in SCLC has been under question, though performed in earlier stage 1 as per the International Guidelines. Some reports document better OS in early stage III too.
Introduction
Small cell lung cancer (SCLC) is an aggressive high-grade neuroendocrine malignancy with high metastatic potential with poor clinical outcomes mostly occurring in 60-80 years. The median overall survival (mOS) for patients with metastatic SCLC receiving standard chemotherapy is ranges between 9–11 months.[1] SCLC iscommonly seen in heavy smokers (depends on amount of tobacco consumption) as an advanced stage disease, it clinically presents with early metastatic spread and good responsiveness to initial therapy, and in most patients, it is consistently followed by relapse. With a chemo resistant disease. The SCLC has location in hilar or perihilar areas, with less than 5% of the cases at peripheral locations. [2] Concomitant inactivation of two tumour suppressors, p53 and RB (encoded by TP53 and RB1, respectively), is found in the vast majority of SCLC cases.[3] SCLC has association with paraneoplastic syndromes such as paraneoplastic endocrinopathies include syndrome of inappropriate anti-diuretic hormone and Cushing syndrome. The features include dermatomyositis, and rarely hyperglycaemia, hypoglycaemia.1–7.4% of patients with cancer can develop a paraneoplastic syndrome.[4] The common cytologic feature is highly cellular aspirates with presence of small blue cells with very scant cytoplasm, loosely arranged or in a syncytial pattern. The nuclear marker Ki-67, which is present in samples is characteristic. Patients with high Ki-67 had a better survival effect than those with low Ki-67, and patients with complete responses had a higher Ki-67 value.[5] The most common symptoms in patients with Extensive stage disease were shortness of breath, fatigue, coughing, chest pain, and nausea/vomiting. Shortness of breath is a fairly constant symptom, although it worsened with exertion and could be exacerbated by coughing.[6] SCLC is easilydiagnosed by cytology. There are a number of pitfalls in the diagnosis of SCLC. These include lack of cytology–histology correlation.A chest x-ray is the most standard imaging test to look for any abnormality within the lung. If abnormality is present, a computed tomography (CT) scan is frequently ordered to reveal the size, shape, and position of any lung tumour. [7]
Therapy for Small Cell Lung Cancer
After theinitial treatment, many patients of SCLC develop recurrent disease, often with additional sites of metastasis. The basic Initial line of therapy includesplatinum–etoposide was found to be associated with the high overall survivalandwas well tolerated. Very few drugs have been provedeffective for second-line treatment of SCLC. Topotecan is a standard second-line choice but is not uniformly used for patients in part due to its modest efficacy and highhematologic toxicity. Overall survival (OS) in patients treated with topotecan is only 26 weeks vs. 14 weeks in patients managed with the best supportive care alone. Single-agent regimens of standard cytotoxic agents, such as paclitaxel, docetaxel, gemcitabine, and vinorelbine, as second line therapiesyield modest results.[8]
Extensive-stage small-cell lung cancer (ES-SCLC) have been significantly improvedby incorporating immune checkpoint inhibitors (ICIs) into platinum-based chemotherapy. Use of antiangiogenic agents, PARP inhibitors (PARPi), lurbinectedin, and anti-DLL3 agents, offering insights into potential future directions in the management of this aggressive cancer. CASPIAN have shown substantial evidence of benefit from adding atezolizumab and durvalumab respectively to chemotherapy, leading to the approval of these check point inhibitors (CPIs) in the frontline setting of ES-SCLC.[9]
|
1. Chemotherapy improves the survival of patients with limited-stage disease (LD) or extensive-stage disease (ED), andit is curative in only a few patients. |
|
2. The combination of platinum and etoposide is the most widely used standard chemotherapeutic regimen. [Level of evidence A1] |
|
3. SCLC is highly radiosensitive and thoracic radiation therapy improves survival of patients with LD and ED tumors. Cranial radiation should also be provided[Level of evidence A1] |
|
4. Clinical trials evaluating new drug regimens or under evaluation |
|
5. For recurrent disease – Chemotherapy & Immunotherapy is evaluated |
Platinum compounds are the mainstay of chemotherapeutic regimens in SCLC patients.[10]
Evaluation of recent therapeutics for refractory or recurrent setting or approved second line drugs : Lurbinectedin is a DNA alkylating agent, which covalently binds to guanine residues in the DNA . It also causes inhibition of RNA-polymerase-II activity.
It is usedfor the treatment of adult patients with metastatic small-cell lung cancer (SCLC) with disease progression on or after platinum-based chemotherapy.
The drug has high volume of distribution, with high penetration to cancerous cells.Its synthetic derivative of trabectedin whichis used to treat refractory, metastatic small cell lung cancer. In an evaluatingtrial ,the median OS of 5.1 months in the second-line cohort and 5.6 months in the third-line setting was found .
In elderly patients, lurbinectedin seems to be superior to the standard of care in terms of both efficacy & safety.
Lurbinectedin leads tolymphopenia , fatigue, chest pain, headache & tumor lysis syndrome.
Indicated for metastatic small cell lung cancer (SCLC) in patients with disease progression on or after platinum-based chemotherapy 3.2 mg/m2 IV q21Days (only if absolute neutrophil count (ANC) ≥1,500 cells/mm3 and platelet count ≥100,000/mm3). [11], [12]
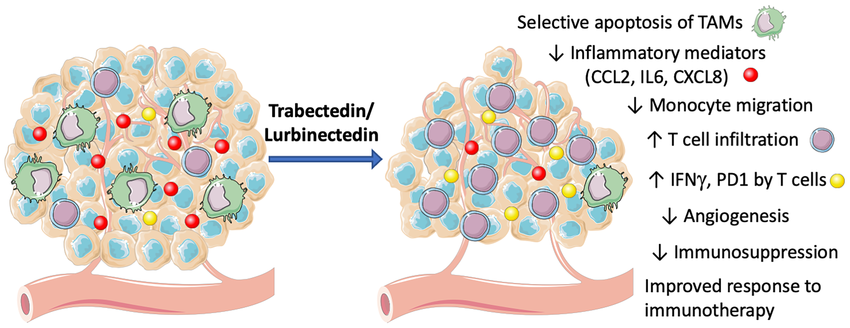
Diagramadopted from
Paola Allavena et al, Effects of the Anti-Tumor Agents Trabectedin and Lurbinectedin on Immune Cells of the Tumor Microenvironment, Frontiers in oncology ,2022
Bcl-2 Inhibition and navitoclax
Navitoclax and Venetoclaxbind to apoptosis suppressor proteins Bcl-2, Bcl-XL, and Bcl-w, which are frequently overexpressed in var Indicated for metastatic small cell lung cancer (SCLC) in patients with disease progression on or after platinum-based chemotherapy
Navitoclax administration (orally bioavailable) includes 150-mg 7-day lead-in dose followed by 250-mg daily dosing with the option to further increase to 325 mg after 14 days.lymphopenia and thrombocytopenia arefrequently observed side effects. [13]
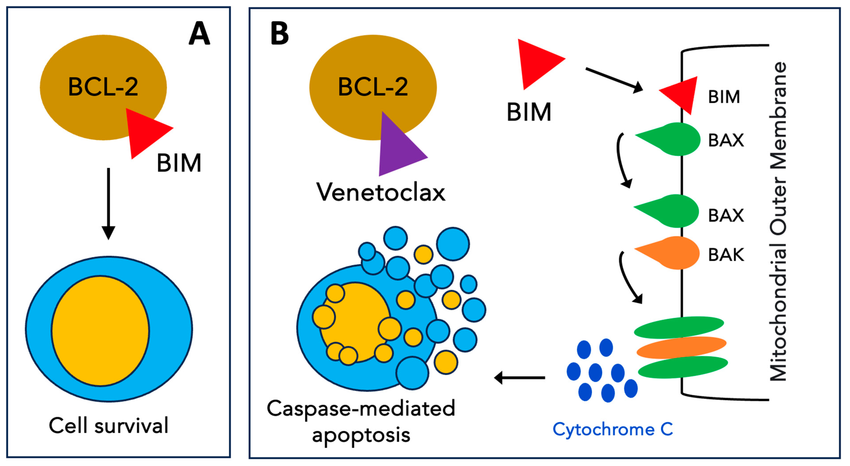
Diagram adopted From
Ramy Rahmé, Venetoclax Combined with Intensive Chemotherapy: A New Hope for Refractory and/or Relapsed Acute Myeloid Leukemia? Journal of clinical medicine January 2024, 13(2):549
Aurora A kinase (AAK) inhibition
Aurora A kinaseis linked to mitosis regulation and is involved in formation of spindle poles. Acts on G2/ M phases of cell.
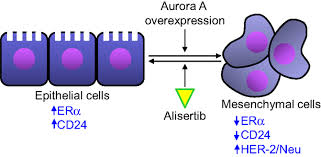
Diagram adopted from
Huifeng Neu, Scientific rationale supporting the clinical development strategy for the investigational Aurora A kinase inhibitor alisertib in cancer, Front. Oncol., 24 August 2015 Alisertib/paclitaxelis used in relapsed or refractory SCLC. Baraseritib a prodrug reduces glucose in cancer cells in dose dependent manner. The maximum tolerated dose of Barsasertibis200 mg, given as a 2-h weekly infusion. Danusertib is another drug [14]
Poly-ADP ribose (PARP) inhibition
PARP inhibitors represent a novel class of anti-cancer therapy and they work by taking advantage of a defect in DNA repair in cancer cells with BRCA mutations and inducing cell death. Talazoparib has been demonstrated to be the most potent PARP trapper followed by niraparib, then olaparib and rucaparib, with veliparib being the least potent trapper. SCLCpatients have high PARP1 protein expression.
The first Chinese study on ES -SCLC was Trident, where safety and efficacy of Olaparib + Durvalumab was evaluated. In this study ORR was 75% and the median PFS was 5.8 months. [15]
DLL-3 Targeting ADCs
DLL3 is atarget identified in tumor-initiating cells which is expressed in more than 80% of patients with SCLC. Rovalpituzumab tesirine is a DLL3-targeted ADC that consists of a humanized DLL3-specific IgG1 monoclonal antibody (mAb),and a protease-cleavable linker that covalently links the antibody to the toxin. Rovalpituzumab tesirine was 0.4 mg/kg every 3 weeks, and the recommended phase 2 dose and schedule were 0.3 mg/kg every 2 weeks .Itshowslinear pharmacokinetics, and a half-life of around 10–14 days. Median overall survival was 5.8 months, and 1-year overall survival was 32% in DLL3 high expressors.The most common side effects aregrade 3 and higher toxicities were thrombocytopenia in 12%, serosal effusions in 11%, and skin reactions in 8%. Skin reactions of any grade areseen in 49%.[16]
Tarlatamab, a bispecific T-cell engager molecule, which binds to both DLL3 and CD3 leading to T-cell–mediated tumor lysis. Tarlatamab demonstrates a manageable safety profile across a wide doserange of 100 mg.
Tarlatamab, administered as a 10-mg dose every 2 weeks, revealedthe objective response rate (ORR)40%. [17]
Cyclin dependent kinase 4/6 (CDK 4/6)
Palbociclib,Abemaciclib inhibit transition from G1 to S phase during the cell cycle via inhibition of CDK4 and CDK6, which they target with varying potency. Palbociclib has similar binding affinity to CDK4/cyclin D3 and CDK6/cyclin D1, whereasabemaciclib have greater binding affinity for CDK4/cyclin D3.
Roniciclib from Bayer AG,is an oral, small-molecule pan–cyclindependent kinase (CDK) inhibitor Roniciclib used in 5mg twice daily or7.5 mg twice dailyBID on a 3 days on/4 days off schedule in 21-day cycles. Common Roniciclib-related adverse events includesnausea (76.6%), fatigue (65.8%), diarrhoea (63.1%), and vomiting (57.7%). [18]
Angiogenesis inhibitors
The addition of bevacizumab to chemotherapy did improve PFS but did not have an impact on OS.
The regimens had Etoposide and Irinotecan combination , Cisplatin + Irinotecan , Paclitaxel or Topotecan with Bevacizumab 7.5 mg/kg dosage[19]
Fibroblast growth factor receptor (FGFR)-Polysomy of chromosome 8
Pazopanib is a commercially available multi-kinase inhibitor with activity at FGFR1 at 800mg dose. Pazopanib maintenance yielded better results for patients who were initially on Etoposide and Platinum Chemotherapy. [20]
Metronomic therapy is atherapy in which anti-cancer drugs are administered in a lower dose than the maximum tolerated dose repetitively over a long period to treatsolid cancers with fewer side effects. Metronomic chemotherapy regimen of cisplatin, etoposide, and irinotecan werecompared to single-agent topotecan in sensitive recurrent SCLC. OS in patients taking the three-drug metronomic regimen was significantly longer than for patients treated with topotecan alone (18.2 vs. 12.5 months). [21]
Anlotinib: is a novel multi‐target small molecule tyrosine kinase inhibitor (TKI) which effectively inhibit the vascular endothelial growth factor (VEGF) receptor, the fibroblast growth factor receptor (FGFR), the platelet‐derived growth factor receptor (PDGFR) α and β and c‐Kit; hence, it has both anti‐angiogenesis and tumor growth inhibition effects. Anlotinib is administered asone cycle of 12 mg daily for 14 days, discontinued for 7 days, and then repeated every 21 days. Anlotinib plus penpulimab had an objective response rate (ORR) of 42.1%and a median overall survival (OS) of 13.0 months. [22]
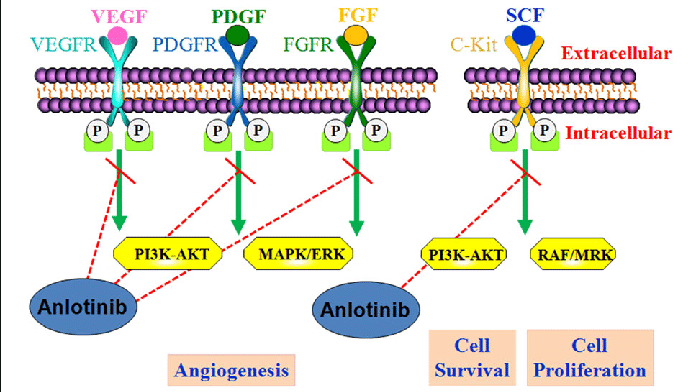
Diagram adopted from
Guoshuang Shen et al, Anlotinib: a novel multi-targeting tyrosine kinase inhibitor in clinical development, , Journal of Hematology & Oncology (2018) 11:120
Somatostatin receptor 2
Somatostatin receptor 2 (SSTR2) is overexpressed in a majority of neuroendocrine neoplasms, including small-cell lung carcinomas. 48% of carcinomas expressed SSTR2. satoreotide, targeting extensive stage small cell lung cancer (ES-SCLC), is being evaluated.[23]
Radiotherapy in SCLC
Chemoimmunotherapy is the first-line treatment for extensive-stage small-cell lung cancer (ES-SCLC). The addition of radiotherapy after first-line treatment for ES-SCLC canfurther improve local control. Radiotherapyis givenonce or twice daily for 5 days / week , for 3 to 7 weeks. Thoracic radiotherapy in addition to prophylactic cranial irradiation canbe considered for all patients with ES-SCLC who respond to chemotherapy. [24]
Surgery in SCLC
The role of surgery in the integrated management of small cell lung cancer remained under investigation with retrospective studies demonstrating 5 year survival of approximately 50% for stage I disease. Lobectomy improved the OS in patients with Stage 11b disease. There was no statistically significant difference in survival between the surgery and the nonsurgery groups in patients with stage IIB disease. Surgery is not option in later stages as the disease spreads rapidly. [25], [26]
Vaccines
GD3 is a cell membrane ganglioside whichhas been shown to be overexpressed in approximately 60% of SCLC tumors , and has been investigated as a potential vaccine target for SCLC The anti-idiotype vaccine to the GD3 ganglioside, BEC-2, has recently been tested in a phase III trial.
(IFN-α) is a cytokine that stimulates immune response and promotes antigen presentation on tumor cells . Two important studies evaluated the role of IFN-alpha in SCLC.[27]
Discussion
The clinical management of small cell lung cancer stillachallenge for clinicians. Poor prognostic factors in SCLC include weight loss, increased age, male sex, elevated lactate dehydrogenase (LDH) and low sodium , syndrome of inappropriate antidiuretic hormone secretion (SIADH) and impaired performance status (PS). The following sequenceis followed for initial and later stages of SCLC.
|
SCLC |
Management |
|
Early Stage |
• Surgeryor Stereotactic Body Radiation Therapy (linear accelerator or proton beam) (only rarely) Chemotherapy |
|
|
• Radiation |
|
|
• Palliative care |
|
Extensive Stage |
• Chemotherapy |
|
|
• Radiation (for symptom amelioration) Immunotherapy |
|
|
• Palliative care |
New treatments are needed in order to improve the prognosis of Extensivestage -SCLC, as median survival with current standard treatment is stillremains 9–10 months from diagnosis. About 60 to 80 percent of SCLC patients respond fairlywell to first-line therapy; however, the majority of SCLC tumors develop resistance to chemotherapy, leading to disease progression. SCLC that comes back after first-line treatment is known as recurrent SCLC. The general survival rate at 5 years for localized SCLC (confined to the lung) is 27%. For SCLC that has spread within the chest area, survival rate at 5 years is 16%.
The first line treatment,etoposide or irinotecan with cisplatin or carboplatin providesbetter response rates.Platinum-etoposide based induction chemotherapy along with surgery in stage I-IIIA SCLC with 3 year survival rates of 74 % for stage I and II and 43% for stage IIIA disease are common observations.
The strategy of combining platinum/doublet and the PD-L1 inhibitor atezolizumab has demonstrated improved outcomes in the front-line setting. The PD-1 inhibitor pembrolizumabhas been voluntarily withdrawn for its indication in patients with metastatic small cell lung cancer (SCLC) after at least 2 prior lines of therapy.
|
• Cause cancer cell death rapidly |
|
• Carry other drugs to tumor & cut off blood flow |
|
• Make cancer cells more responsive to immune system |
|
• Prevent / block interrupt cell growth |
|
• Target defects in cancer cells |
Immunotherapy has provided a brief response, although the prognosis remains poor, even after the advent of new drugs. With the advent of new therapies, the extension of life span in SCLC isslightly improved .5%–10% of patients will achieve long-term disease remission. Second and subsequent lines of therapy rarely produce durable responses.
Source of Funding
None.
Conflict of Interest
None.
References
- AF, Keane F. Current standards for clinical management of small cell lung cancer. Transl Lung Cancer Res. 2018;7(1):69-79. [Google Scholar]
- MR, Bota-Rabassedas N, Wistuba I. Pathology and Classification of SCLC. Cancers. 2021;13(4). [Google Scholar] [Crossref]
- CR, Brambilla E, CF, Sage J. Small-cell lung cancer. Nat Rev Dis Primers. 2021;7(1). [Google Scholar] [Crossref]
- Soomro Z, Youssef M, Yust-Katz S, Jalali A, Patel A, Mandel J. Paraneoplastic syndromes in small cell lung cancer. J Thorac Dis. 2020;12(10):6253-63. [Google Scholar]
- Sagmen S, Dogan C, Comert S, Kiral N, Parmaksiz E, Fidan A. The importance of Ki-67 proliferation index in small cell lung cancer. Eur Respir J. 2020;56. [Google Scholar] [Crossref]
- Bebb D, Murray C, Giannopoulou A, Felip E. Symptoms and Experiences with Small Cell Lung Cancer: A Mixed Methods Study of Patients and Caregivers. Pulm Ther. 2023;9(3):435-50. [Google Scholar]
- Travis W. Update on small cell carcinoma and its differentiation from squamous cell carcinoma and other non-small cell carcinomas. Mod Pathol. 2012;25(1):18-30. [Google Scholar]
- Yang S, Zhang Z, Wang Q. Emerging therapies for small cell lung cancer. J Hematol Oncol. 2019;12(1). [Google Scholar] [Crossref]
- Gomezrandulfe I, Leporati R, Gupta B, Liu S, Califano R. Recent advances and future strategies in first-line treatment of ES-SCLC. Eur J Cancer. 2024;200. [Google Scholar] [Crossref]
- Giunta E, Addeo A, Rizzo A, Banna G. First-Line Treatment for Advanced SCLC: What Is Left Behind and Beyond Chemoimmunotherapy. Front Med (Lausanne). 2022;9. [Google Scholar] [Crossref]
- MS, Farid S, Liu S. Drugs in development for small cell lung cancer. J Thorac Dis. 2020;12(10):6298-307. [Google Scholar]
- Manzo A, VS, Carillio G, Palumbo G, Montanino A, Sandomenico C. Lurbinectedin in small cell lung cancer. Front Oncol. 2022;12. [Google Scholar] [Crossref]
- Rudin C, Hann C, Garon E, Oliveira, M, Bonomi P, Camidge D. Phase II study of single-agent navitoclax (ABT-263) and biomarker correlates in patients with relapsed small cell lung cancer. Clin Cancer Res. 2012;18(11):3163-9. [Google Scholar]
- Du R, Huang C, Liu K, Li X, Dong Z. Targeting AURKA in Cancer: molecular mechanisms and opportunities for Cancer therapy. Mol Cancer. 2021;20(1). [Google Scholar] [Crossref]
- Barayan R, Ran X, Lok B. PARP inhibitors for small cell lung cancer and their potential for integration into current treatment approaches. J Thorac Dis. 2020;12(10):6240-52. [Google Scholar]
- Rudin C, Reck M, Johnson M, Blackhall F, Hann C, Chih-Hsin J. Emerging therapies targeting the delta-like ligand 3 (DLL3) in small cell lung cancer. J Hematol Oncol. 2023;16. [Google Scholar] [Crossref]
- Ahn M, Cho BC, EF, Korantzis I, Ohashi K, Majem M. Tarlatamab for Patients with Previously Treated Small-Cell Lung Cancer. N Engl J Med. 2023;389(22):2063-75. [Google Scholar]
- Fassl A, Geng Y, Sicinski P. CDK4 and CDK6 kinases: from basic science to cancer therapy. Science. 2022;375(6577). [Google Scholar] [Crossref]
- Hamilton G, Rath B, Voutsina A, Mavroudis D, Georgoulias V. Targeting angiogenesis in small cell lung cancer. Transl Lung Cancer Res. 2017;5(4):389-400. [Google Scholar]
- Sun J. Pazopanib maintenance after first-line etoposide and platinum chemotherapy in patients with extensive disease small-cell lung cancer: a multicentre, randomised, placebo-controlled Phase II study (KCSG-LU12-07). Br J Cancer. 2018;118(5):648-53. [Google Scholar]
- Goto K, Ohe Y, Shibata T, Seto T, Takahashi T, Nakagawa K. Combined chemotherapy with cisplatin, etoposide, and irinotecan versus topotecan alone as second-line treatment for patients with sensitive relapsed small-cell lung cancer (JCOG0605): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2016;17(8):1147-57. [Google Scholar]
- Zhang C. Efficacy and safety of anlotinib plus penpulimab as second-line treatment for small cell lung cancer: A multicenter, open-label, single-arm phase II trial. Cancer Pathogenesis and Therapy. 2024. [Google Scholar]
- Park H, Tseng S, Sholl L, Hatabu H, Awad M, Nishino M. Molecular Characterization and Therapeutic Approaches to Small Cell Lung Cancer: Imaging Implications. Radiology. 2022;305(3):512-25. [Google Scholar]
- Tjong M, Mak D, Shahi J, Li G, Chen H, Louie A. Current Management and Progress in Radiotherapy for Small Cell Lung Cancer. Front Oncol. 2020;10. [Google Scholar] [Crossref]
- EK, Prokakis C, Karanikolas M, Apostolakis E, Dougenis D. Current role of surgery in small cell lung carcinoma. J Cardiothorac Surg. 2009;4. [Google Scholar] [Crossref]
- Huang Z, Liu Y, Wang S, Ai K, Zhang P. Kaixing Ai & Peng Zhang, Surgery for stage IIB-IIIB small cell lung cancer. World J Surg Oncol. 2023;21. [Google Scholar] [Crossref]
- HM, Induru R, Jalal S. Novel therapies in small cell lung cancer. Transl Lung Cancer Res. 2015;4(5):533-44. [Google Scholar]
- Abstract
- Introduction
- Therapy for Small Cell Lung Cancer
- Diagramadopted from
- Bcl-2 Inhibition and navitoclax
- Diagram adopted From
- Aurora A kinase (AAK) inhibition
- Diagram adopted from
- Poly-ADP ribose (PARP) inhibition
- DLL-3 Targeting ADCs
- Cyclin dependent kinase 4/6 (CDK 4/6)
- Angiogenesis inhibitors
- Fibroblast growth factor receptor (FGFR)-Polysomy of chromosome 8
- Diagram adopted from
- Somatostatin receptor 2
- Radiotherapy in SCLC
- Surgery in SCLC
- Vaccines
- Discussion
- Source of Funding
- Conflict of Interest
- References
How to Cite This Article
Vancouver
Chaudhry S, Trailokya A. Conceptual therapeutics for small cell lung cancer [Internet]. IP Indian J Immunol Respir Med. 2024 [cited 2025 Nov 02];9(3):98-103. Available from: https://doi.org/10.18231/j.ijirm.2024.020
APA
Chaudhry, S., Trailokya, A. (2024). Conceptual therapeutics for small cell lung cancer. IP Indian J Immunol Respir Med, 9(3), 98-103. https://doi.org/10.18231/j.ijirm.2024.020
MLA
Chaudhry, Sunil, Trailokya, Abhijit. "Conceptual therapeutics for small cell lung cancer." IP Indian J Immunol Respir Med, vol. 9, no. 3, 2024, pp. 98-103. https://doi.org/10.18231/j.ijirm.2024.020
Chicago
Chaudhry, S., Trailokya, A.. "Conceptual therapeutics for small cell lung cancer." IP Indian J Immunol Respir Med 9, no. 3 (2024): 98-103. https://doi.org/10.18231/j.ijirm.2024.020
