Introduction
Paraquat is a highly corrosive, green liquid with a strong odour. It is a commonly used herbicide because of its low cost. It is not absorbed through intact skin or by inhalation. Absorption through the gut is rapid. After absorption, it enters the systemic circulation and causes multi-organ damage. 1 In the lung, the most common manifestation is pulmonary fibrosis. Other rare manifestations are pneumo-mediastinum and pneumothorax, which are abnormal accumulation of air in the mediastinum and pleura, respectively.2 Among various poisoning, paraquat has higher mortality, and it accounts for 13% of all-cause death related to poisoning.3 Here, we describe a suicidal paraquat poisoning case with multi-organ involvement presenting initially as subcutaneous emphysema, pneumo-mediastinum, and pneumoperitoneum with acute respiratory failure.
Case Summary
An 18-year-old male, student presented with acute onset difficulty in breathing with neck and facial swelling for one day. Breathlessness was sudden in onset, not associated with wheeze, and with no postural or diurnal variation. He had a history of intermittent fever, nausea, and vomiting for three days. He had no history of trauma, insect bite, reduced urine output, bilateral leg swelling or physical exertion. On examination, the patient was tachypnoeic, drowsy, oriented to time, place, and person. Facial puffiness and neck bulging were noted on inspection. He had extensive subcutaneous emphysema on palpation from the scalp to the abdomen.
He had room air saturation of 90% and maintained 95% saturation at FiO2 of 30%. He had a blood pressure of 110/70 mm of Hg and a pulse rate of about 95/min. Arterial blood gas analysis suggested type 2 respiratory failure (PH-7.14, PCO2-87.9 mmHg, PO2-50 mmHg, HCO3-24 mEq/L). Chest auscultation revealed fine bilateral crepitation with decreased intensity on the right side. Ultrasound thorax showed absent lung sliding on the right side. Hence, pneumothorax was suspected and an emergency tube thoracostomy was done on the right 4th intercostal space in the mid-axillary line, after which his room air saturation improved to 93%. The patient was intubated and kept on a mechanical ventilator in pressure control mode to protect the airway.
Post-intubation, patient arterial blood gas analysis was showed: PH-7.42, PCO2-36 mmHg, PO2-57mmHg, HCO3-24 mEq/L. Chest X-ray antero-posterior view was suggestive of extensive subcutaneous emphysema along with pneumo-mediastinum (Figure 1a). High-Resolution Computed tomography (HRCT) thorax showed gross subcutaneous emphysema extending into myofascial planes of the neck, face, chest wall and upper abdomen, gross pneumo-mediastinum and pneumo-pericardium with mild bilateral pneumothorax. Air foci were also seen in bilateral lungs along the bronchial tree, suggestive of pulmonary interstitial emphysema and limited sections of the upper abdomen shows subdiaphragmatic air foci, suggestive of pneumo-peritoneum with right side intercostal tube in situ (Figure 2 a and b). Hence, the left side tube thoracostomy was also done in view of persistent pneumomediastinum. Oral contrast computed tomography of the abdomen with thorax was done to rule out oesophageal rupture, which was normal. The patient was weaned and extubated to improve conscious and subcutaneous emphysema after two days.
Even after all the investigations, the causes of the above signs and symptoms were unknown. Hence, the patient inquired about the previous history, revealing paraquat consumption two days before symptom onset. Hence diagnosis was made. However, oral decontamination and treatment for paraquat poisoning were not given because of unconfirmed diagnoses. A urine dithionite test confirmed paraquat poisoning, which was positive. Laboratory data on admission showed Haemoglobin (Hb)-15.3g/dl, total white blood cell (WBC) - 14×109 cells (neutrophils 90%, lymphocytes 3.5%, monocytes 6% and eosinophils 0.1%). Over the hospital stay, the total count increased up to 45×109 cells, and it was neutrophil predominant (neutrophils 90%, lymphocytes 5.1%, monocytes 4.7% and eosinophils 0.1%). Blood culture showed Serratia marcescens growth, and he was started on an oral minocycline tablet. Creatinine kinase and creatinine kinase MB were 992 IU/litre and 33 IU/litre. Amylase (939 IU/litre) and Lipase (1539IU/litre) were also high. Total bilirubin increased progressively. (Table 1).
The patient developed respiratory distress with worsening in room air saturation after two days of extubation. Hence, the patient was re-intubated. The patient was treated with N Acetylcysteine, antibiotics and adequate supportive measures. Methylprednisolone and cyclophosphamide, which are considered to prevent pulmonary fibrosis, were not started given inadequate evidence as the patient was on the fourth day of the poisoning. Subcutaneous emphysema, pneumothorax and pneumo-mediastinum resolved over the hospital stay. Hence, intercostal tube drainage was removed. However, the patient developed acute respiratory distress syndrome (Figure 1b) and expired on the 17th day of poison intake. Written informed consent was obtained from the patients parents for publication of the case details and images.
Figure 1
a: Chest X-ray antero-posterior view showed bilateral subcutaneous emphysema with pneumomediastinum; b: Chest X-ray showed resolution of subcutaneous emphysema with bilateral non homogenous opacity.
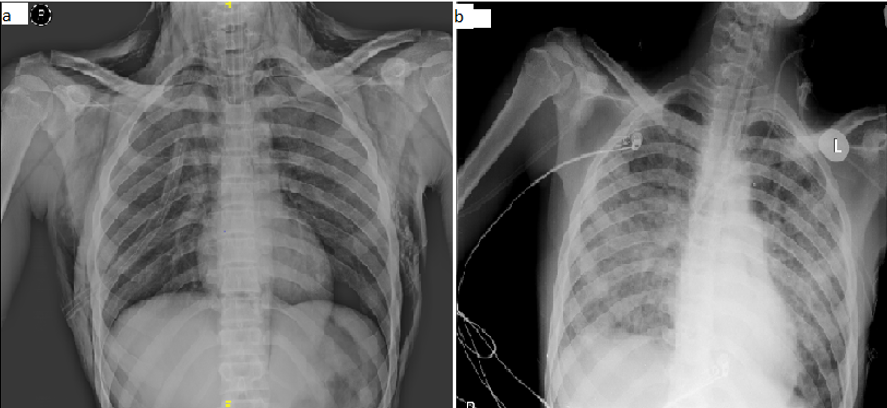
Figure 2
a: High-Resolution Computed tomography (HRCT) Thorax showed gross subcutaneous emphysema with pneumomediastinum with bilateral pneumothorax; b: Computed tomography abdomen showed in subdiaphragmatic air collection suggestive of pneumoperitoneum.
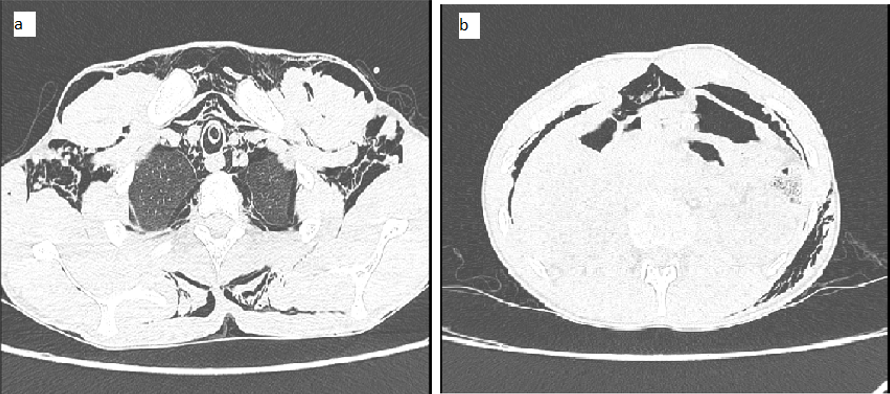
Table 1
Renal function and Liver function test of the patient during hospital stay
Discussion
Paraquat is a highly corrosive substance used in agriculture as a pesticide. In South Asia, mainly in India, the most common poisoning is paraquat poison. The typical route of poisoning is swallowing.4 After ingestion, it will distribute to other tissue and achieve maximum tissue level within six hours. Local and systemic side effects of paraquat poisoning are due to superoxide radicals, nitrate radicals and other reactive oxygen species. 5 In humans, the lethal dose of paraquat is 30 mg/kg, corresponding to 8-10 mL of the 24% solution. 6 It does not undergo significant metabolism and is eliminated mainly by the kidneys. So, the ingested paraquat will appear in urine within 24 hours. Nevertheless, renal function is impaired in severe paraquat poisoning, leading to slower elimination. 7
In the lung, paraquat enters into type 1 and 2 alveolar epithelial cells by active transport and releasing reactive oxygen species, damaging the cell membrane. As the lung concentration is 10-20 times greater than the plasma, the lung is most commonly affected, leading to pulmonary fibrosis. 8 Reactive oxygen species produced by paraquat metabolism lead to hepatotoxicity and nephrotoxicity. Death in paraquat poisoning is mainly due to lung injury. In 1990, Daisley and Barton described the first presentation of spontaneous pneumothorax, subcutaneous emphysema and pneumomediastinum due to paraquat poisoning. 9 The reasons for this presentation may be attributed to the corrosive effect of paraquat leading to oesophageal erosion and perforation or due to air leakage from the injured alveoli. Paraquat poisoning destroys the type 2 pneumocytes leading to a rise in surface tension, secondary atelectasis, sub-pleural bleb formation and rupture, producing pneumothorax and emphysema. Air travels along the vascular sheath and connective tissue plane, leading to pneumomediastinum. The presence of pneumothorax and pneumo-mediastinum are specific determinators of increased mortality in paraquat poisoning.2
Symptoms and signs usually manifest within 12 hours of paraquat ingestion. Diagnosis is usually clear from history but challenging to differentiate from unknown agricultural poisoning. A urine dithionite test can be used for confirmation of paraquat poisoning in this situation. Gastrointestinal decontamination is recommended to limit systemic exposure when the diagnosis is made. 10 Other procedures like hemoperfusion or haemodialysis followed by continuous hemodiafiltration may be beneficial if started within four hours of ingestion. 10 There is no proven antidote for paraquat poisoning to date. Oxygen therapy aggravates lung injury. Hence oxygen should be avoided or used minimally according to the requirement.11 N-Acetylcysteine, antioxidants (Vitamin E, C) and paraquat antibodies were beneficial in paraquat poisoning. 11 In moderate to severe paraquat poisoning, corticosteroids and cyclophosphamide can be used to prevent inflammation and pulmonary fibrosis. 12 In case of acute renal failure, the patient should undergo haemodialysis. Lung transplantation can be used in paraquat poisoning, but the evidence is lacking. 13
Conclusions
Spontaneous pneumo-mediastinum, subcutaneous emphysema and pneumo-mediastinum are rarely seen in paraquat poisoning but are associated with a high mortality index. When pneumo-mediastinum is present without recognized causes, paraquat poisoning should be considered a differential diagnosis even if there is no clear history of intentional poison intake. Early general measures for poisoning should be considered, as full recovery is possible even though it is associated with a grave prognosis.
Acknowledgement
The authors would like to acknowledge the services rendered by Dr. Zeenathalam Nadaf, Dr. Karthik, Dr. Jayapritha in patient care and management.
