Introduction
Corona Virus Disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) characterized as a pandemic by the World Health Organization.
Accumulating evidence suggests that immune dysfunction and cytokine dysregulation results in cytokine storm in severe COVID-19 cases.1, 2, 3 Persistent elevated levels of inflammatory cytokines may lead to an increased risk of vascular hyperpermeability, multiorgan failure, and eventually death overtime.4
CD6 is a co-stimulatory molecule required for optimal T-cell stimulation by the antigen-presenting cells.5 The CD6 pathway contributes to Th1 activation and differentiation of T-cells, promoting a proinflammatory response. Itolizumab, a humanized recombinant anti-CD6 monoclonal antibody of IgG1 isotype, is a selective T-cell co-stimulation modulator that targets the extracellular, scavenger receptor cysteine-rich distal domain 1 of CD6 on T-cells.6 It has been found to inhibit T-cell proliferation induced in the presence of ALCAM and excess IL-2 and down regulates the phosphorylation of intracellular proteins implicated in the CD6-mediated activation pathways and reduces IFN-γ, IL-6, and TNF-α production, leading to a reduction in the T-cell infiltration at the sites of inflammation.7 However, it does not result in T-regulatory cell depletion.
Considering the lack of standard treatments for COVID-19, immunomodulatory agents like Itolizumab, used to reduce systemic inflammation, may constitute a possible treatment option. Itolizumab has been approved in India for the treatment of moderate-to-severe psoriasis7 and has shown promising results when used in chronic plaque psoriasis,8 associated psoriatic arthritis9, 10 and rheumatoid arthritis.11 In COVID-19 patients, Itolizumab can significantly result in reduction of hyperinflammation by controlling the pro-inflammatory ‘cytokine storm’ syndrome. On 10th July 2020, Drug Controller General of India (DCGI), approved Itolizumab for restricted emergency use in India for treatment of cytokine release syndrome (CRS) in moderate to severe acute respiratory distress syndrome (ARDS) due to COVID-19.
An informed consent for publication could not be taken from the patient because of the pandemic situation and constraints in resources and access. However, a no objection certificate was obtained from hospital ethics committee on 9th October, 2020. EC NO: ECR/388/Inst/MH/2013/RR-19. Due efforts were made to conceal information on patient identity.
Case Report
Here we report a case of a COVID-19 positive, 69 years old, high risk patient with dual comorbidities of diabetes and hypertension treated with Itolizumab along with best supportive care.
The patient was hospitalized on 22nd May 2020 with a history of fever, cough and fatigue. There was no history of diarrhea, headache, or sore throat. Significant medical history included diabetes and hypertension. The patient was on Metformin 500 mg plus Glimepiride 4 mg twice daily, Amlodipine 5 mg twice daily, Telmisartan 40 mg twice daily and a Statin 20 mg once a day.
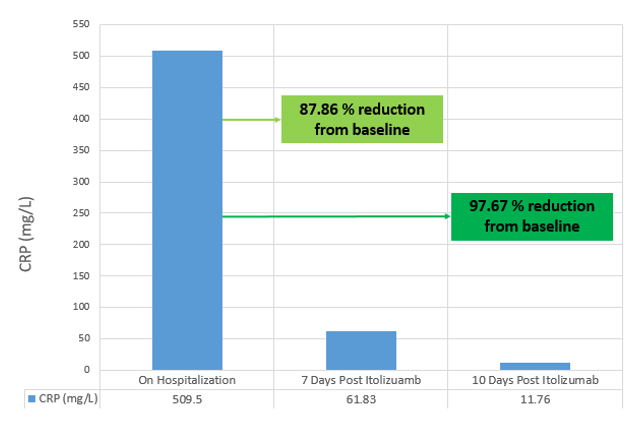
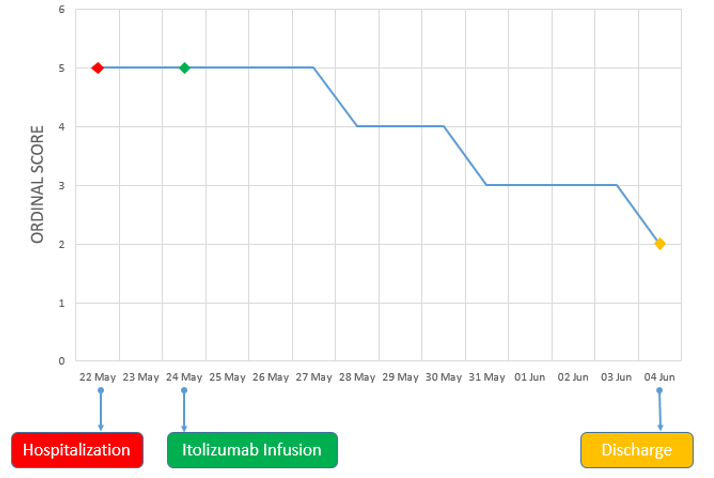
On examination, his body temperature was 38.3°C, blood pressure was 100/70 mmHg, respiratory rate 26/min, FiO2 rate of 90% and body weight of 68 kg. The patient was positive for SARS-CoV2 infection (RT-PCR). Hematology, liver function tests and serum creatinine were within normal limits. Inflammatory markers, serum ferritin (399.9 ng/ml) and CRP (509.5 mg/L) were elevated. His SpO2 level was 80% and a chest X-ray revealed ground glass opacities at the basal and hilar regions, suggestive of severe acute respiratory syndrome (Figure 1A). His clinical status score was 5 based on 9-point ordinal scale.12
The patient was treated with oxygen (15 L/min) via a high flow rebreathing (HFR) mask along with best supportive care including antipyretics, Vitamin C, Remdesivir, Hydroxychloroquine and Steroids. His oxygen saturation was maintained at 85%. Itolizumab (1.6 mg/kg) was administered as an intravenous infusion on 24th May 2020 (Day 1) for a duration of five to six hours after premedication with Hydrocortisone 100 mg IV, 30 min prior, and was well tolerated. The patient continued to receive oxygen via HFR mask at the same rate (15 L/min).
On Days 3 and 4 of Itolizumab infusion, the patient continued to be on oxygen via HFR mask (15 L/min) and maintained a SpO2 90% - 92%. From Days 5 to 7, the patient improved clinically, was shifted to nasal prong for oxygen (2-4 L/min) and maintained SpO2 of 92% - 95%. On Day 7, there was a reduction in inflammatory marker CRP from baseline (87.86% reduction). The ordinal scale for clinical improvement score improved to score 4 on Day 5 and remained so until Day 7.
On Day 8, oxygen requirement further reduced, and the patient was off oxygen. On Day 9, there was further clinical improvement and the chest x-ray revealed reduction of opacities at the basal and hilar regions with evidence of healing (Figure 1B). The ordinal scale for clinical improvement score improved to 3 on Day 8 and was at the same level until discharge (day 12). CRP showed significant decrease of 97.69% from baseline to Day 10 (Figure 2). On Day 11, chest x-ray showed signs of healing with fibrosis (Figure 1C).
Over the course of treatment, the clinical improvement correlated well with changes in inflammatory markers and chest X-ray findings. The patient was discharged on 4th June 2020 (Day 12 of Itolizumab administration).
Key laboratory parameters are provided in Table 1.
Figure 1
Chest X-ray images; (A): On 22nd May 2020 (Upon Hospitalization), (B): On 1st June 2020 (Day 9 post Itolizumab infusion), (C): On 3rd June 2020 (Day 11 post Itolizumab infusion)
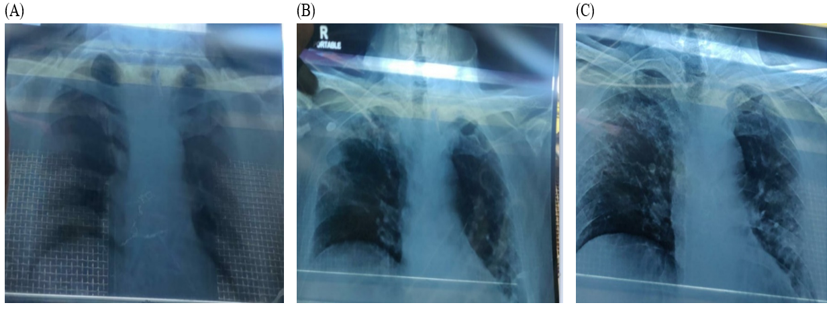
Table 1
Key laboratory parameters
Discussion
The patient presented at the hospital with a history of fever, cough and fatigue and his medical history included diabetes and hypertension. In agreement with the comprehensive analysis by Yong Hu et al. (2020), hypertension and diabetes are the most prevalent comorbidities and are associated with the severity of COVID-19.13 Age, presence of underlying diseases and secondary infections along with low lymphocyte count and elevated serum levels of CRP, D-dimers, ferritin, cardiac troponin and IL-6 are generally considered in risk stratification to predict severe and fatal COVID-19 in hospitalized patients.14, 15 Elevated CRP is considered as an early marker to predict disease progression and severity. This patient also presented with very high CRP level of 509.5 mg/ L, which was alarming along with high serum ferritin (399.9ng /ml). After treatment, CRP level showed a significant decline of 87.86% and 97.69% from baseline on day 7 and day 10, respectively. The absolute lymphocyte counts were maintained throughout the duration of hospitalization.
The predominant CT findings of COVID-19 infection are bilateral, peripheral and basal predominant ground-glass opacity, consolidation, or both.16, 17 As per previous reports, improvement in chest CT is usually seen in 14 day time.17 In this patient, initial examination of chest X-ray revealed ground glass opacities at the basal and hilar regions which reduced by Day 9 of treatment.
Reported median time for weaning off oxygen therapy was 6 days and it ranged from 2 to 35 days.18 An improvement of two points on an eight-point ordinal scale was observed and patient was off oxygen on day 8 post treatment (Figure 3). The patient clinically improved and was discharged from the hospital at day 12.
The limitation of this case study is that we could not measure the various other inflammatory markers like IL-6, D-dimer and Ferritin, which could have further added value to these positive clinical and radiological findings. Further research in a clinical trial setting is warranted to validate these findings and analyse efficacy and safety of Itolizumab combined with best supportive in COVID-19 disease.