Introduction
Obstructive sleep apnea (OSA) is a sleep disorder characterized by recurrent upper airway obstruction during sleep, leading to intermittent breathing pauses alveolar hypoventilation and intermittent arousal from sleep. These in turn result in imbalances in arterial blood gas levels and acid base levels.1 OSA is associated with insulin resistance, hypertension and cardiovascular morbidities. Worldwide OSA prevalence ranges between 17–24% for men and 5–9% for women.2, 3, 4, 5, 6, 7 The prevalence of OSA in Delhi was found to be 13.7% among men and 3.6% among women.8
Recent studies have focused on understanding the etiology for exponential rise and wide range in OSA prevalence (14-55%) and the association of OSA with metabolic syndrome, obesity and diabetes mellitus. Metabolic syndrome (MetS) is a group of several cardiovascular risk factors like central obesity, dyslipidemia, higher blood pressure levels and blood glucose levels, with raises the prothrombotic and inflammatory states.2, 3, 4, 5, 6, 7 The prevalence of obesity and MetS is rapidly increasing in India leading to increased mortality and morbidity due to CVD and Type 2 Diabetes mellitus (T2DM). About 1/3rd of India’s urban population has MetS.2, 3, 4, 5, 6, 7 OSA has been independently associated with insulin resistance, implying OSA may be an important factor for T2DM and MetS.2, 3, 4, 5, 6, 7
Polysomnography is the gold standard test for diagnosis of OSA, estimation of its severity and measurement of treatment response.9, 10 Earlier detection of obstructive sleep apnea in MetS and initiation of Continuous Positive Airway Pressure (CPAP) therapy in the earlier stages of the disease are known to improve not only OSA, but also metabolic syndrome components. Hence, early detection and management of OSA in Metabolic syndrome is essential. A majority (80%) of OSA cases remain undiagnosed.2, 3, 4, 5, 6, 7 Patients with untreated OSA suffer from a wide range of complications like diabetes, hypertension, glaucoma and various cardiovascular adverse events, that lead to increased healthcare expenditures and high consumption of healthcare resources. Whereas, the timely and effective management of OSA can substantially minimize the complications, morbidity profile as well as out of pocket expenditures.1, 5, 7 As the data on OSA in metabolic syndrome in India is scarce, the present study was conducted with the aim and objectives: a) To estimate the prevalence of obstructive sleep apnea among metabolic syndrome patients by conducting polysomnography and b) to study the factors associated with OSA among these patients.
Materials and Methods
This Hospital based cross-sectional study was conducted at the out-patient departments of Department of Pulmonary Medicine and General Medicine of B.J. Government Medical College and Sassoon General Hospitals, Pune, Maharashtra. The study was done for a period of 2 years from 1st January 2015 to 31st December 2016. Approval from Institutional Ethics committee was obtained prior to commencement of the study. Patients visiting the out-patient department of Pulmonary Medicine and General Medicine were screened for metabolic syndrome and those who were diagnosed to be suffering from Metabolic syndrome according to 2005. After explaining the purpose of the study and obtaining their written informed consent, patients were included in the study.
International diabetes federation
Criteria were considered for the study.11, 12
Patients aged ≥ 18 years, of either sex, who were diagnosed to be suffering from metabolic syndrome as per ‘the 2005 International Diabetes Federation (IDF) criteria for metabolic syndrome’ and who gave informed consent, were included in this study. According to the 2005 IDF criteria, metabolic syndrome is diagnosed in a patient having:
Central obesity (waist circumference [WC] ≥ 90 cm in males and ≥ 80 cm in females)
With any two of the following:
Serum triglycerides (TG) ≥ 150 mg/dL, or specific treatment for lipid abnormality
Serum High Density Lipoprotein Cholesterol (HDL-c) less than 40 mg/dL in males and less than 50 mg/dL in females, or specific treatment for lipid abnormality.
Blood pressure (BP) in supine position (after 10 min rest): systolic BP ≥ 130 mmHg or diastolic BP ≥ 85 mmHg, or on the treatment of previously diagnosed hypertension.
Fasting blood sugar (FBS) ≥ 100 mg/dL or previously diagnosed type 2 diabetes mellitus.
Exclusion criteria
Consisted of critically ill patients, Patients with end stage organ disease and malignancy, hypothyroidism, pregnant women, non-cooperative and unwilling patients, and patients aged less than 18 years.
Patients with metabolic syndrome who fulfilled the study eligibility criteria were included in the study after explaining the purpose of the study and obtaining their informed consent. Thorough clinical history and examination was performed. Socio-demographic profile of participants were noted in a pre-formed, semi-structured proforma, by interview method. Patients’ BMI, blood pressure (recorded after at least 5 minutes of rest in both arms sitting/supine position) and waist circumference (measured in a horizontal plane midway between the inferior margin of the ribs and superior border of the iliac crest) were measured.
Blood samples of 5ml collected after 12 hours overnight fasting for the measurement of lipid profile, fasting blood glucose. The patients were screened for symptoms of OSA. Epworth sleepiness scale was used to screen for Excessive Daytime Sleepiness (EDS). Physical examination was performed to look for upper airway anatomy (Mallampati grading was used). Patients were subjected to overnight Polysomnography.
Equipment
The study was conducted by a 32 channel Polysomnography (PSG) machine (Embla S7000, Elders pvt. Ltd) which is used to perform online sleep studies in hospital under pulmonologist’s supervision. The machine was a 32 channel Polysomnography (Embla S7000, Elders pvt. Ltd). Measures of body habitus were recorded by standard anthropometric methods. PSG consisted of continuous polygraphic recording from surface leads for EEG, EOG, EMG, ECG, pressure transducers for nasal airflow, thoracic and abdominal impedance belts for respiratory efforts, pulse oximeter for oxyhemoglobin level, snoring sensor, sensors for leg and sleep position.
OSA severity grades were based on the apnea-hypopnea index (AHI), which is defined as number of apnea and hypopnea episodes per hour of sleep. Mild, moderate and severe OSA grades were assigned for apnea-hypopnea index scores of 5-14/h, 15-30/h and more than 30 episodes per hour respectively. 13, 14
Upper airway anatomy is involved in the pathogenesis of OSA. 15, 16 Modified Mallampati classification is a standard method to evaluate the patient’s airway for intubation, performed pre-operatively for predicting a difficult endotracheal intubation. it is a useful sign in the clinical assessment of OSA. It is based on the morphology of the oropharynx, which is also a simple assessment tool for OSA. 16 Patients were made to sit at eye level to the investigator, with mouth fully open and tongue maximally protruded and patients were asked not to phonate. The class is then graded based on the visibility of the airway structures. Class I: Soft palate, fauces, uvula, and pillars are seen, Class II: Soft palate, fauces, and uvula are seen, Class III: Soft palate and base of uvula seen, Class IV: Soft palate not visible.15, 16
Epworth Sleepiness Scale (ESS) is a self-administered questionnaire which assesses daytime functioning including concentration levels, work performance and sleepiness.17 ESS scale has 8 questions with a 4-point scale ranging from 0-3, wherein, ‘0’ indicates ‘would never nod off’, while ‘3’ indicates a strong chance of nodding off. The ESS scale questions elicit the information in 8 different situations of daily life. 17 ESS total score is attained by the adding all the individual item scores. The ESS score range is 0 to 24. Higher ESS total scores indicate greater degree of daytime sleepiness in respondent.
Statistical analysis
Data was compiled in MS Office Excel spreadsheet and analyzed using SPSS V:20 (Statistical Package for Social Service). Qualitative data variables were expressed in frequency and percentages. Quantitative data variables expressed in mean and standard deviation. Chi-square test and independent student t test were applied to find the association of obstructive sleep apnea with metabolic syndrome variables (qualitative and quantitative variables respectively). Association of metabolic syndrome parameters with OSA severity grades was estimated by applying One-way ANOVA test. Associations with p value of < 0.05 were considered be statistically significant.
Results
A total of 60 metabolic syndrome patients who fulfilled the study eligibility criteria, participated in the study. The prevalence of obstructive Sleep Apnea in metabolic syndrome was found to be 73.3%. (Table 1 ) 26.7% (16 patients) of OSA patients had Mallampati grade I, 43.3% (26 patients) had Grade II and 16.7% (10 patients) had grade III Mallampati grades.
Table 2 describes factors associated with obstructive sleep apnea. OSA patient group belonged to older age group (50.68±14.15 years) when compared to non-OSA group (43.13± 9.71 years) and this age difference was found to be statistically significant. Majority of males (81.0%) had OSA when compared to females (55.6%), and this association of sex with metabolic syndrome was found to be statistically significant. Excessive daytime sleepiness (EDS) and snoring were significantly associated with OSA, wherein, a higher percentage of study patients who reported to have EDS and snoring (84.2% and 84.8% respectively) had OSA, when compared to those patients without the symptoms of Excessive daytime sleepiness and snoring (54.5% and 35.7% respectively). Similarly, Epworth Sleepiness Scale (ESS) score of ≥ 10 was found to be significantly associated with OSA, wherein 86.1% of patients with ESS ≥ 10 had OSA when compared to 54.2% of patients with ESS score of <10. Body Mass Index was found to be significantly associated with OSA, wherein majority of 84.4% obese patients (BMI: ≥ 30) suffered from Obstructive Sleep Apnea when compared to those with normal or low BMI levels (45.5% OSA among Low BMI group patients and 70.6% OSA among normal BMI group patients). Majority of MetS patients with serum triglyceride levels of ≥ 150mg% (83.3%) had obstructive sleep apnoea when compared with patients with serum triglyceride levels of <150mg% (58.3%) and this association was found to be statistically significant. Although majority of patients with T2DM (80.8%) and hypertension (77.5%) were found to be suffering from OSA, these associations were not found to be statistically significant.
Among the MetS study population, patients suffering from Obstructive Sleep Apnea had significantly larger Waist Circumference (105.09 ± 9.86 cm) and Neck Circumference (38.82 ± 3.91 cm) when compared to that of Non-OSA group (96 ± 4.95 cm waist circumference and 36.63 ± 1.84cm neck circumference). Similarly, OSA patients had significantly higher BMI levels (33.09± 8.38Kg/m2) when compared to BMI levels of Non-OSA patients (26.75±5.946 Kg/m2). Serum triglyceride levels among OSA group was higher (181.50±38.63mg/dL) and serum high density lipoprotein (HDL) cholesterol levels were significantly lower (42.77±6.32mg/dL) among OSA group compared to non-OSA group (TG: 162.63±10.57mg/dL and HDL-C 52.38±3.38 mg/dL) and these associations were found to be statistically significant. Other parameters such as systolic blood pressure, diastolic blood pressure and fasting blood glucose levels were higher among OSA group (136.18±29.65 mm Hg, 84.91±6.810 mm Hg and 114.68±18.26 mg/dL respectively) when compared with those of non-OSA group [(135.21±17.68 mm Hg, 84.50±7.31 mm Hg and 105.25±18.02 mg/dL respectively)]. But these differences in SBP, DBP and FBS were not found to be significantly significant. (Table 3)
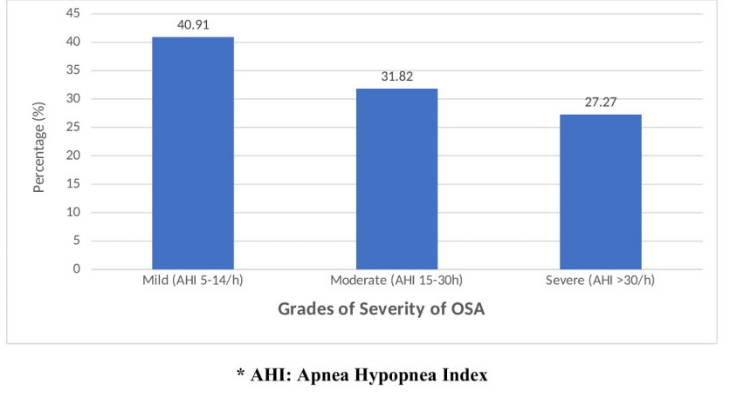
A 40.91% of OSA patients had mild OSA with AHI of 5-14 episodes per hour, 31.82% of OSA patients had moderate grade OSA (AHI: 5-30 episodes per hour) and 27.27% of patients had severe grade OSA with an AHI of > 30 episodes per hour. (Figure 1). Table 4 describes the association of metabolic syndrome parameters with severity of obstructive sleep apnea. The OSA severity increased with increasing age. The average age of patients with mild OSA was 45.67 ± 14.3 years, moderate OSA was 46.29 ± 14.4 years and severe OSA group was 48.33 ± 12.2 years. Accordingly, the average levels of body mass index (35.75 ± 8.3 Kg/m2), waist circumference (110.75 ± 11.9 cm), neck circumference (40.58 ± 5.2cms) and serum triglycerides (186.67±42.5mg/dL) were highest among the severe OSA group when compared with mild, moderate and no OSA groups. These associations were found to be statistically significant.
The average levels of serum HDL cholesterol (38±2.6mg/dL) among patients with severe OSA were significantly lesser when compared with that of mild, moderate and no OSA groups (46.89 ± 6.4, 41.57 ± 4.9 and 52.38 ± 3.3mg/dL respectively).
Table 1
Prevalence of Obstructive Sleep Apnoea (OSA) in Metabolic Syndrome patients
|
OSA |
Number of patients |
Percentage (%) |
|
Present |
44 |
73.3 |
|
Absent |
16 |
26.7 |
|
Total |
60 |
100.0 |
Table 2
Factors associated with OSA
Table 3
Association of metabolic syndrome parameters with OSA among the two study groups
Table 4
Associationof metabolic syndrome parameters with OSA severity grades
Discussion
Metabolic syndrome in Indian subcontinent has shown an increasing trend with a wide range of prevalence (11-40%).12, 18 ‘Syndrome Z’ terminology was coined by Wilcox et al., to describe clinical condition with a combination of metabolic syndrome (Syndrome X) and OSA.12, 19 Untreated and severe grades of OSA lead to endocrine hormonal imbalances due to increased systemic inflammation and sympathetic over-activity, thereby worsening metabolic syndrome.
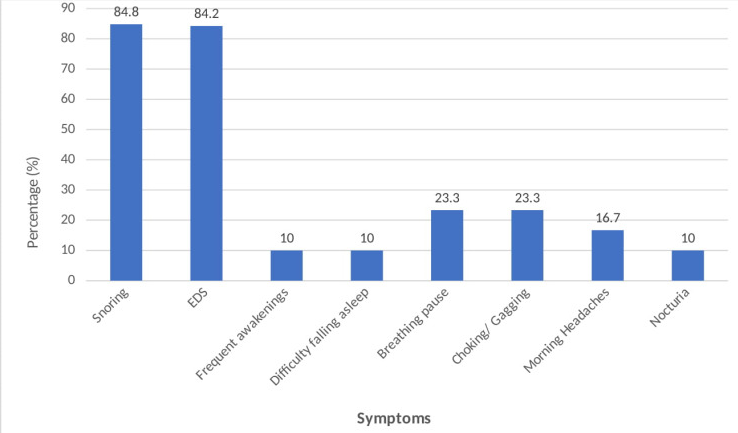
The present study was conducted at a tertiary care teaching hospital at Pune, Maharashtra. It was found that the prevalence of obstructive sleep apnea among metabolic syndrome patients was 73.3%. Similar higher rates of OSA in MetS are found in studies conducted by Soin D. et al.12, 20, 21 In OSA patients, intermittent and repeated upper airway obstruction during sleep due to recurrent collapse of the pharyngeal airway, result in hypopnoeic or apnoeic episodes despite ongoing breathing efforts. These breathing disruptions lead to intermittent blood gas disturbances (hypercapnia and hypoxemia) and surges of sympathetic activation. These recurrent episodes lead to altered breathing cycles and disturbed sleep.22 Snoring was the predominant symptom reported among OSA patient group (76.7%). Other symptoms reported were excessive daytime sleepiness (63.3%), breathing pause and choking (23.3%). (Figure 1). Similar results were found in studies conducted by Young et al., and Udwadia et al.,23, 24.
The age of youngest patient in present study was 28 years, while oldest patient was 75 years old. The average age of patients in the study was 48.67±13.46 years. The study documented a significantly higher preponderance of OSA to males (81.0%) compared to females (55.6%) and to older age group (50.68±14.15 years). Similar results of higher prevalence of OSA with male gender and older age group are found in majority of studies.3, 8, 10, 12, 21 This gender related protective effect among females, decreases in post-menopausal phase in those who are not receiving hormone replacement therapy. In addition to the lesser likelihood of women being evaluated and treated for OSA, effect of sex hormones, craniofacial morphology, pattern of fat deposition, differences in upper airway shape and genioglossal muscle activity during the wake state and control of ventilation, have been proposed to account for a higher male risk of OSA.23
Obesity, more importantly, central obesity is more crucial for OSA than either age or sex, as it has greater impact on the upper airway function due to associated increased neck circumference, resulting in smaller upper airway area and greater compression on airway while sleeping. There is graded increase in OSA prevalence and OSA severity with increasing The Body Mass Index (BMI). Central obesity leads to resistance to leptin, thereby production of leptin is enhanced. Leptin increases the likelihood of obstructive sleep apnea, whereas weight reduction leads to reduction of apnea-hypopnea episodes per hour.8, 9, 10, 12, 21, 24 Accordingly, present study has shown that obesity, larger waist and neck circumferences were found to be significantly associated with OSA and OSA severity (84.4% obese patients had OSA. (Table 2)
Severe and untreated obstructive sleep apnea leads to intermittent hypoxia, endothelial dysfunction, sympathetic hyperactivity and aldosterone excess. These factors lead to spikes in blood pressure levels and thereby aggravate hypertension. Increased sympathetic activity also leads to endocrine organ dysfunction and inhibition release of hormones. Pancreatic insulin secretion, hepatic glucose production and adipocyte regulation of energy balance are severely affected due to this high sympathetic activity. Thus, an independent association is found between OSA and insulin resistance irrespective of diabetes mellitus. 25 The present study noted a trend of association of higher levels of systolic and diastolic blood pressures and fasting blood glucose severe OSA grades, but these associations did not reach statistical significance. Similar results are found in studies conducted by Agrawal S, Udwadia et al., and Soin et al.,8, 12, 24.
Chronic intermittent hypoxia which is a characteristic feature of OSA, is independently associated with dyslipidemia, oxidative and immune-inflammatory alterations. Chronic intermittent hypoxia also leads to generation of stearoyl-coenzyme A desaturase-1 and reactive oxygen species, peroxidation of lipids and sympathetic system dysfunction. Systemic inflammatory markers like cytokines (IL-1) may alter LDL metabolism by altering endothelial cell cholesterol metabolism. Thus, OSA is independently associated with dyslipidemia and serum triglycerides, LDL cholesterol are found to be directly related with higher AHI levels, whereas HDL cholesterol are inversely related to AHI levels. 2, 10, 26 The present study found similar trend of significantly higher serum triglyceride levels (186.67±42.5mg/dL) and significantly lesser serum HDL cholesterol levels (38±2.6 mg/dL)in severe OSA cases. (Table 3, Table 4)
Conclusions
The present study showed that there is a very high prevalence of obstructive sleep apnea among patients with metabolic syndrome. Central obesity, with higher body mass index levels, larger waist circumference and neck circumferences, were the striking features of OSA patients. And these higher levels were found to be significantly associated with severe grades of OSA. Dyslipidemia is another major parameter that was found to be highly predominant among OSA patients. OSA group had significantly higher levels serum triglyceride and lower levels of HDL cholesterol. Also, the present study documented a trend of increased fasting blood sugar levels, increased systolic and diastolic blood pressure levels associated with moderate and severe OSA grades (severe OSA group), but these associations were not statistically significant. Thus, the present study has reiterated the fact that OSA is associated with higher levels of metabolic dysfunction. The coexistence of both OSA and MetS, within the same patient has shown a greater derangement of metabolic functions and disruption of endocrine homeostasis.12, 21
Recommendations
Treatment of obstructive sleep apnoea with Continuous Positive Airway Pressure (CPAP) therapy in the earlier stages of the disease is known to be effective in controlling apnoea, daytime sleepiness. Scientific literature has shown that CPAP therapy also provides effective control on metabolic syndrome components like diabetes mellitus and hypertension, by reducing insulin resistance, reducing blood pressure and blood glucose levels, by controlling dyslipidemia, thereby reducing the adverse cardiovascular events.5, 7, 12, 21, 26 There is a need for greater focus on early diagnosis of obstructive sleep apnea by the physicians at all levels, by using a simple self-administered Epsworth Sleepiness Scale, further to be followed by the overnight polysomnography, which is the gold standard for diagnosing OSA. This screening and early detection of OSA among general population and specifically among metabolic syndrome patients, can go a long way in improving the longevity and improve the health outcomes among patients with metabolic syndrome and obstructive sleep apnea.
Strengths of the study
The study has highlighted the raised prevalence of OSA in metabolic syndrome. The study has shown the levels of metabolic dysfunctions that exist in the various grades of severity of OSA.