- Visibility 70 Views
- Downloads 10 Downloads
- DOI 10.18231/j.ijirm.2020.044
-
CrossMark
- Citation
ANA negative/sero negative systemic lupus erythematosus with diffuse alveolar haemorrhage: A diagnostic difficulty
- Author Details:
-
Ravindra Chari M
-
Pratap Upadhya *
-
RamyaPriya A
-
Pampa ch.toi
-
Vishnukanth G
Introduction
Antinuclear antibody (ANA) negative lupus is an important subset of the systemic lupus erythematosus (SLE) disease spectrum. Occurrence of true ANA-negative SLE is a rare clinical entity. Even if these serological markers are absent, these patients will have similar clinical manifestations to their seropositive counterparts, hence the term “seronegative” SLE is given.
We report a case of seronegative SLE associated with diffuse alveolar haemorrhage (DAH) and respiratory failure. It also emphasizes the spectrum of severity like pulmonary hypertension, skin ulcers, deep vein thrombosis (DVT) and demonstrates how early diagnosis and therapy can change the course of the disease.
Case Report
A 45 years female with recent onset systemic hypertension was presented with dry cough and breathlessness for one month worsened over one week. General examination revealed mild pallor and pitting pedal oedema extending till mid-calf along with crusted 1 x 1 cm non healing ulcer on right ankle near lateral malleolus. There was no cyanosis, icterus, clubbing and lymphadenopathy. Her vital were- pulse rate-94/min, BP-130/80mm Hg, Spo2-96% room air, respiratory rate-24cycles/min.
She was started on broad spectrum antibiotics in view of clinical suspicion of community acquired pneumonia. Within two days of hospital admission, the patient developed hypoxia (SpO2-90%) needing supplementary oxygen (O2) (Two litres of O2 via nasal cannula).
Initial laboratory evaluation revealed anaemia (haemoglobin of 9.6 g/dl) with total leukocyte count (TLC) of 8,110/mm3 and platelet count 91,000/ mm3. After two days, haemoglobin was dropped to 7.7g/dl and platelet count dropped to 48,000/ mm3 with TLC of 7,640/ mm3. Erythrocyte sedimentation rate and C-reactive protein were normal. Her sputum and blood cultures were normal. HIV, liver function tests and thyroid function tests were normal. Urine analysis showed proteinuria of 2+ and 24 hr urine protein was 756 mg/day without any bacteria or RBC/ WBC casts.
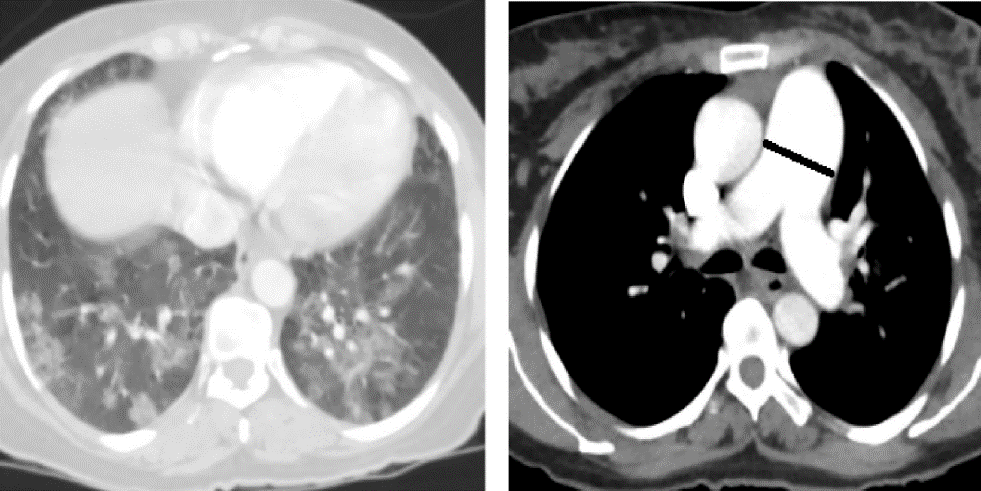
Chest X-ray suggested nonhomogeneous opacity in bilateral lower zones with cardiomegaly. CT pulmonary angiogram (CTPA) showed dilated main pulmonary artery (diameter of 3.41cm), bilateral diffuse mosaic pattern and consolidation predominantly in lower lobes with Left Internal jugular vein (IJV) thrombus [[Figure 1]]. Doppler USG of bilateral upper and lower limbs showed Right popliteal and Left IJV thrombosis. 2D-echocardiography showed moderate tricuspid regurgitation with right ventricular enlargement.
There was no clinical and laboratory clues for infection. Autoimmune serological work up and biopsy from ulcer was done to rule out immunological causes. Autoimmune serology work up showed ANA by enzyme-linked immunosorbent assay (ELISA), ANA human epithelial cells (HEp-2) substrate and anti-dsDNA antibody (ELISA) Were twice negative. Antibodies against extractable nuclear antigens (ENA) to Sm, Scl70, SSA, SSB, Jo-1, Ro were negative. Perinuclear and Cytoplasmic Anti-nuclear Cytoplasmic Antibody (P-ANCA and C-ANCA), rheumatoid factor, anti-cyclic citrullinated peptide antibodies were also negative. lupus anticoagulant (LA), Anti-Beta 2 glycoprotein antibody (anti-𝛽-2 gp Ab) and anti-cardiolipin antibodies (ACL) for APLA (antiphospholipid antibody) syndrome were present. Complement levels were low which includes C3- 29mg/dl, C4- 4mg/dl and total <3 U/ml.
Based on radiology and immunological workup, immune mediated lung injury was suspected and diagnostic bronchoscopy was performed. Her bronchoalveolar lavage (BAL) from right lower lobe (RLL) showed sequentially increasing haemorrhagic lavage consistent with DAH. [[Figure 2]]
Skin biopsy from ulcer reported as lymphatic inflammatory infiltration in the dermal vessel walls and endothelial cell swelling suggestive of lymphocytic vasculitis. Direct immunofluorescence suggestive of IgG and IgA 2+ granular deposits at dermo-epidermal junction and inter epidermal keratinocytes. C3- negative. Features were consistent with immune complex deposition disease.
As clinical (skin ulcers), laboratory (thrombocytopenia, proteinuria) and immunology (low C3 and C4, APLA antibodies) parameters were fulfilling the Systemic Lupus International Collaborating Clinics(SLICC) criteria, primary diagnosis of SLE was established though ANA was negative.
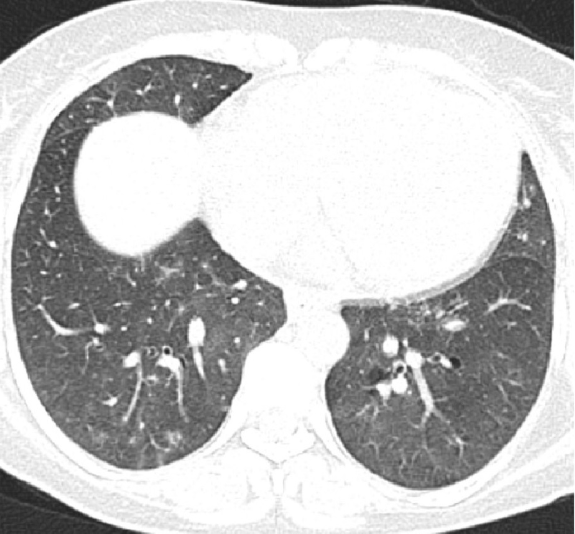
Multiple DVTs were attributed to secondary APLA (SLE induced) as evidenced by LA, anti-𝛽-2 gp Ab, ACL antibodies which were second time positive after 12 weeks of follow up. Proteinuria, thrombocytopenia, pulmonary hypertension, and skin ulceration were attributed to SLE induced vasculitis and thrombotic thrombocytopenic purpura (TTP). As the patient had multiple issues, and critical thrombocytopenia, renal biopsy was deferred and immediately started on oral prednisolone one mg/kg. Oxygen supplementation was continued. Serial platelet count monitoring showed increasing trends and skin ulcer started healing. Antihypertensives along with anticoagulation were started with target INR goal of 2–2.5. Patient showed clinical and radiological resolution [[Figure 3]], hence completely weaned off from oxygen supplementation. At the time of follow up after one month, skin ulcer was healed with little scarring. Platelet count improved to 2,06,000/ mm3, with near total resolution radiological opacities and proteinuria.
Based on the above findings, the final diagnosis of ANA negative /seronegative SLE with APS presenting as DAH, pulmonary hypertension with thrombocytopenia, skin ulcers was made.
Discussion
SLE is a multisystem disease with unknown aetiology and is a great mimicker of other diseases. The diagnosis of SLE usually made based on clinical and laboratory parameters using American College of Rheumatology (ACR) which required four out of 11 clinical criteria and/or Systemic Lupus International Collaborating Clinics(SLICC) criteria which requires four of 17 criteria, including at least one clinical criterion and one immunologic criterion or biopsy-proven lupus nephritis in order to classify as having SLE[1].
This discussion is chiefly focused on the features of our case described.
SLE and ANA
ANA is widely used as a screening modality for diagnosing clinically suspected SLE. SLE is a heterogeneous spectrum of disease that includes ANA positive lupus and ANA negative or seronegative lupus[2]. Seronegative lupus was initially described in the 1970s. Subsequently, the reporting of ANA-negative lupus was minimized with the introduction of human cell lines in place of mouse tissue substrate as a source for ANA analysis. According to various studies, ANA negative SLE is very rare, with an incidence of <4% among all lupus patients[3]. If clinical suspicion is strong, negative serological tests do not exclude SLE as this test may become positive in further stages even after years. Whatever may be the ANA status at the time of disease presentation, the diagnosis of SLE can be made if the patient fulfils the ACR/SLICC criteria.
SLE and DAH
SLE can involve lung in various forms. Among all, DAH is a life-threatening clinicopathological syndrome where haemorrhage happens from pulmonary microcirculation. Pulmonary capillaritis is the most frequent histopathological pattern rather than bland haemorrhage. DAH can be associated with any condition that leads to inflammatory changes causing capillaritis. Rheumatological disorders are the most common causes of pulmonary capillaritis. DAH as a manifestation of systemic lupus erythematosus (SLE) occurs in 1% to 5% of SLE cases[4].
DAH can present with vague respiratory symptoms including that of dyspnoea and cough with or without haemoptysis, which is one of the alarming symptoms of DAH. Our case similarly presented with dyspnoea and dry cough without significant haemoptysis as it can be absent in one third of the cases. Hence, a high index of suspicion is the key for diagnosing DAH. Various multivariate analysis showed low serum C3 complement factors and thrombocytopenia predisposes to DAH. Both these factors were seen in our case. Diagnosis of DAH requires FOB guided BAL with evidence of sequentially increasing haemorrhagic return of BAL washings. Intact erythrocytes in macrophages with hemosiderin laden macrophages are the clues for diagnosis but it is not pathognomic. Lung biopsies are considered as the gold standard for connective tissue disease (CTD) induced DAH, have become obsolete with the emergence of radiology and serological antibodies. If DAH is secondary to autoimmune antibodies as in primary APLA or in SLE, DAH is likely to be severe. In such cases early and prompt recognition is critically important as mortality reaches 50-95%[5].
SLE and Thrombocytopenia
SLE thrombocytopenia can be because of multiple reasons. Out of all, Peripheral destruction of Platelets being the most common cause. Thrombocytopenia with generalized lupus is referred as TTP-like syndrome and without generalized lupus is referred as bonafide TTP. A similar syndrome can also occur in the presence of antiphospholipid syndrome as ‘extra criteria’ manifestation. Thrombocytopenia is an independent risk factor for increased mortality in SLE[6]. Mild thrombocytopenia may require only observation. If platelet counts <50 × 109/L or thrombocytopenia associated with bleeding, severe bruising corticosteroids are the treatment of choice. Azathioprine can be used as a steroid-sparing agent. Cyclosporine, cyclophosphamide, Intravenous immunoglobulins (IVIG), stem cell transplantation, and splenectomy can be used in refractory cases.
SLE and Pulmonary Artery Hypertension
SLE is the second most common systemic autoimmune disease with concurrent pulmonary arterial hypertension (PAH) after progressive systemic sclerosis. The prevalence of PAH in SLE is < 4% when the diagnosis is defined on the gold standard investigation, namely right heart catheterization (RHC)[7]. In the vast majority of patients SLE diagnosis can precedes PAH. Very rarely, the onset of PAH may lead to lupus diagnosis. The main pathology of SLE-PAH is immune complex-mediated vasculitis with vascular intima and media thickening. Other mechanisms include hypoxia secondary to lung disease such as pulmonary fibrosis, in situ thrombosis secondary to anti-phospholipid antibodies, veno-occlusive disease and when there is imbalance between vasoconstrictors (endothelin- 1, thromboxane A2) vasodilators (prostacyclin). Though the gold standard is RHC, diagnosis can be obtained through Doppler echocardiography and CT which are non-invasive and easily accessible. The CT diagnosis of PAH was done based on enlarged pulmonary artery diameter greater than 29 mm, which is greater than ascending aorta at the same level.[8] This diameter must be measured in the axial plane at the bifurcation, orthogonal to the long axis of the pulmonary artery. Apart from PAH, CT can also diagnose ILD and thrombotic diseases. Our case was diagnosed to have pulmonary hypertension based on CT criteria and Doppler echo.
SLE and skin
Though pathogenesis is unclear, the second most common clinical presentation of SLE is skin damage[9]. This can be associated with less or without severe systemic organ damage. SLE-skin involvement further divided into acute, subacute, chronic, and intermittent. Histopathological features include hyperkeratosis, epidermal atrophy, liquefactive degeneration at epidermal basal-cell, and infiltration of mononuclear cells around perivascular and perifollicular areas suggestive of small vessel cutaneous leukocytoclastic vasculitis. The most common form of vasculitis is cutaneous small vessel vasculitis which can present in several morphological variants including ulcers. Skin deposited IgG is a major pathological factor to trigger skin injury in SLE patients[10]. Immunohistochemistry demonstrates immune complex deposition containing IgG, IgM, and complement at the dermo-epidermal junction.
Skin biopsy from the ankle of our case showed lymphocytic vasculitis with IgG and IgA 2+ granular deposits at dermo-epidermal junction consistent with SLE induced skin ulcers.
SLE and APLA
The antiphospholipid syndrome (APS) is defined by the occurrence of thrombosis, often multiple, and pregnancy morbidity (abortions, foetal deaths and premature births) along with the presence of antiphospholipid LA, anti-𝛽-2 gp Ab, ACL antibodies. The APS can be found in patients without another definable condition (primary APS) or with other diseases, mainly SLE (secondary APS). DVT of the lower extremities is the most commonly reported manifestation in APS. Additionally, thrombosis can also be seen in arms, subclavian and jugular veins.
SLE itself can be considered as a risk factor for thrombosis. In another spectrum, 40% of patients with SLE have APS, but < 40% of them will have thrombotic manifestations. In the evaluation of the thrombotic risk in SLE patients, APS should be taken into account along with additional risk factors, such as older age, traditional cardiovascular risk factors, smoking, oral contraceptives, pregnancy. In the management of unprovoked thrombotic events, arterial events, or the presence of APS support prolonged anticoagulation like our case.

Conclusions
SLE is a heterogeneous disease with a wide spectrum of disease severity. Although a positive ANA is a sensitive and screening marker of SLE, ANA negative SLE also do exist as a subgroup of SLE. It is important for the clinicians to be aware that ANA need not to present always in patients with significant systemic disease. This should be considered when diagnosing and treating patients with suspected SLE.
Acknowledgement
Dr. S. Vinod Kumar, Professor of Pulmonary Medicine, for guidance and material support.
Conflict of Interests
None.
Source of Funding
None.
References
- M Petri, A M Orbai, G S Alarcon, C Gordon, J T Merrill, P R Fortin. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum 2012. [Google Scholar]
- Morris Reichlin. ANA negative systemic lupus erythematosus sera revisited serologically. Lupus 2000. [Google Scholar]
- L.S. Cross, A. Aslam, S.A. Misbah. Antinuclear antibody-negative lupus as a distinct diagnostic entity—does it no longer exist?. QJM 2004. [Google Scholar]
- Jean-François Cordier, Vincent Cottin. Alveolar Hemorrhage in Vasculitis: Primary and Secondary. Semin Respir Crit Care Med 2011. [Google Scholar]
- Edna P. Schwab, H. Ralph Schumacher, Bruce Freundlich, Peter E. Callegari. Pulmonary alveolar hemorrhage in systemic lupus erythematosus. Semin Arthritis Rheum 1993. [Google Scholar]
- C C Mok, K W Lee, C T Ho, C S Lau, R W Wong. A prospective study of survival and prognostic indicators of systemic lupus erythematosus in a southern Chinese population. Rheumatol (Oxford) 2000. [Google Scholar]
- Xiaoqin Yang, Jack Mardekian, Kafi N. Sanders, Marko A. Mychaskiw, Joseph Thomas. Prevalence of pulmonary arterial hypertension in patients with connective tissue diseases: a systematic review of the literature. Clin Rheumatol 2013. [Google Scholar]
- Aletta Ann Frazier, Jeffrey R. Galvin, Teri J. Franks, Melissa L. Rosado-de-Christenson. From the Archives of the AFIP. Radio Graphics 2000. [Google Scholar]
- Guo-Min Deng, George C. Tsokos. Pathogenesis and targeted treatment of skin injury in SLE. Nat Rev Rheumatol 2015. [Google Scholar]
- Guo-Min Deng. Pathogenesis of Skin Injury of Systemic Lupus Erythematosus. Curr Rheumatol Rep 2018. [Google Scholar]
