Introduction
Asthma is a heterogeneous disease characterized by chronic airway inflammation with dominant physiological features of systemic inflammation and reversible airflow narrowing1. The clinical presentations include wheezing, shortness of breath, chest tightness and cough that may vary over time and in intensity, together with variable expiratory airflow limitation. As indicated by WHO, in excess of 300 million individuals around the world experience the ill effects of asthma and this number is rising2, 3, 4. Globally, deaths from this condition have reached over 180,000 every year. India has an estimated 15-20 million asthmatics. Besides local inflammation, there are evidences of systemic inflammation is also present in bronchial asthma by persistent sub-acute inflammation of the airways with hyper-responsiveness, severe inflammation, predispose patients to disease exacerbation and even death. Pathogenesis of asthma are infiltration of such inflammatory cells as eosinophils, basophils, and CD4 + lymphocytes. C-reactive protein (CRP) is a well known inflammatory marker synthesized by hepatocytes 5.
Studies have shown increased levels of systemic markers like plasma fibrinogen, serum amyloid A and high sensitivity CRP in patients with bronchial asthma. Systemic inflammations such as diabetes, cardiovascular diseases, collagen vascular diseases, malignancies, and obesity are increased by these biomarkers. Recent availability of assays for high sensitivity C-reactive protein (hsCRP) can simply and inexpensively measure CRP in acute phase and help in the assessment of systemic inflammation6. Treatment efficacy is assessed based on clinical examination, pulmonary function test and suppression of inflammation with appropriate treatment with reduction of serum hsCRP, protein and serum amyloid A (SAA) is a clinically useful marker of inflammation level.7, 8 Hence, an attempt was made to find out the response of the serum levels of hs-CRP of asthmatic patients with and without inhaled corticosteroid (ICS) treatment were compared as a systemic and airway inflammatory marker was evaluated, in a tertiary care center Department of pulmonary medicine, SRM Medical College Hospital and Research Centre, Potheri, Kattankulathur, Kancheepuram (D.T), Tamilnadu.
Materials and Methods
The aim of our investigation was to survey the degrees of hsCRP in asthmatic patients, with the targets to assess the serum levels of hsCRP in asthmatic patients and to correlate the hsCRP in asthmatic patients with or without inhaled corticosteroids treatment, and to observe the relationship of hsCRP levels to clinical records of asthma.
Study setting
The study was carried out in the Department of Pulmonary Medicine, SRM Medical College Hospital and Research Centre. All the patients who satisfied the inclusion criteria attending the pulmonary medicine of OPD were included in the observational study.
Study design
It is a comparative-descriptive and observational study, done during the period of April 2014 – June 2015 with the prior approval from the Institutional Review Board of SRM Medical College Hospital and Research Centre. Total 80 patients (both old and newly diagnosed cases) were selected for the study and were grouped in to 3 groups of patients viz,
Group-A: Bronchial asthma patients with steroid naïve or not using inhaled corticosteroids and
Group-B: Bronchial asthma patients using inhaled corticosteroids.
Group-C: Normal controls
Inclusion criteria
Patients of age 18 to > 50 years, both male and female gender who are diagnosed with clinical symptoms of asthma with a criteria of spirometric analysis results showing a12% increase and 200 ml increase in FEV 1 post bronchodilator.
Exclusion criteria
Patients with age less than 18 and more than 50 were excluded from the study. Further exclusion criteria includes, current smokers, recent or current infection, patients diagnosed with COPD / respiratory infection within the month preceding the study, history suggestive of any systemic inflammatory disease, obesity, coronary heart disease, diabetes, heart failure, history of venous thromboembolism, kidney disease, liver disease, malignancy and rheumatologic illness9.
Normal volunteers
The normal volunteers recruited in to the investigation as controls based on the willingness and with a criterion that none of them had any past history of lung or hypersensitive ailment and were not utilizing any drug. At the time of recruitment, they all underwent full clinical examination, pulmonary function tests, and blood sampling. The control group volunteers had normal lung function tests (FEV1>80%) and normal IgE level. Those with high IgE were considered as atopic asthma.
Methods
Patients diagnosed with bronchial asthma present to pulmonary medicine OPD, those who satisfy the inclusion criteria were recruited into the study. The data of such recruited patients were recorded in the proforma which includes demographic profile, their familial history and physical examination such as cardinal symptoms of respiratory system, physical examination of respiratory, cardiovascular, abdominal systems and treatment history allergy history if any. In addition the diagnostic work up which includes chest x ray, total and differential leukocyte count, and pulmonary function test. Blood samples were collected from such patients and also from controls and sent for high sensitivity CRP analysis the levels were noted10.
Estimation of high-sensitivity C-reactive protein
Samples of peripheral venous blood were aspirated (2ml) and centrifuged, the serum was separated and subjected for the hsCRP estimation immune-turbidimetry assay. Unlike cardiac conditions, there is no specific cutoff level or threshold level in bronchial asthma. There is a proportional documented increase in hsCRP levels in relationship to severity of bronchial asthma. The test gives results in 25 minutes with sensitivity down to 0.04 mg/L. The American Heart Association and U.S. Centers for Disease Control and Prevention have defined risk groups as low risk (less than 1.0 mg/L), average risk (1.0 to 3.0 mg/L), and high risk (above 3.0 mg/L was taken into account10.
Pulmonary function test
A Pulmonary function test was done to find out FEV1 and FEV1/forced vital capacity (FVC) using computerized Medikro SpiroStar spirometry systems in the Department of Pulmonary Medicine, SRM Medical College Hospital and Research Centre 11, 12.
Statistical methods
Statistical analysis was done in all inclusion criteria subjects. The results were plotted in the Microsoft Office Excel worksheet and were analyzed. Statistical analysis was performed using SPSS v.13. Descriptive statistics done were percentage analysis, cross tabulation analysis and chi square test and Spearmann’s coefficient.
Results
Gender distribution
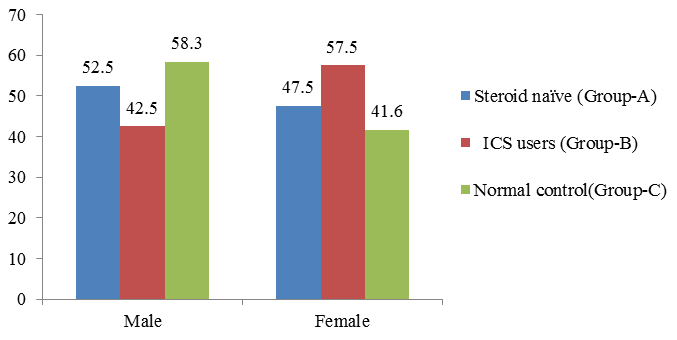
A total of 80 patients with asthma as test group and 24 normal people as control group were recruited in the present study. Of them, 40 patients are Steroid naïve (Group A) with male patients 47.5% and 52.5% are females. Inhaled corticosteroid (grouo-B) males are 42.5 % and females are 57.5 %. Among 24 normal control group recruited for the study, 58.3% are male and 41.3 % are female (Table 1 Figure 1).
Mean age
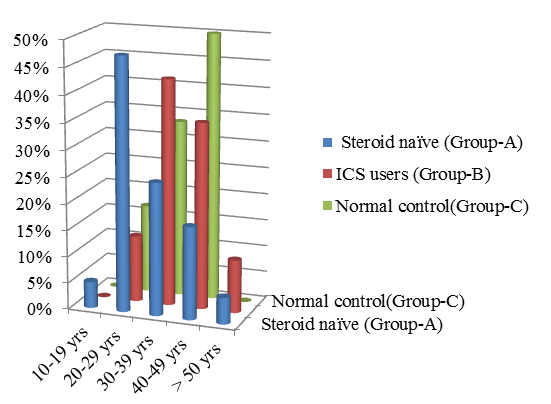
The age distribution of steroid naïve Steroid (ICS) users are shown in the Table 2 Figure 2. The overall mean age of 80 asthmatic patients is 34.8 yrs out of which about 47.5% of the steroid naïve patients belong to age group 20-29 yrs and whereas most of patients who use ICS belong to age group 30-39 years.
Distribution of breathlessness and seasonal variation of symptoms
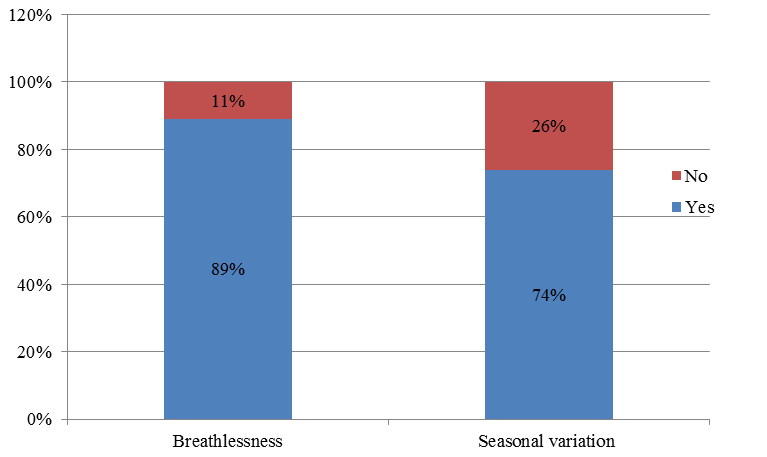
Out of 80 patients, breathlessness was present in about 71 patients (89%) and breathlessness was not a symptom in 9 patients (11%). Seasonal variation of symptoms in which 59 patients (74%) had seasonal variation symptoms of cough, breathlessness, wheezing and chest tightness and 21 patients (26%) have no such seasonal variation symptoms (Figure 3).
Family history of bronchial asthma
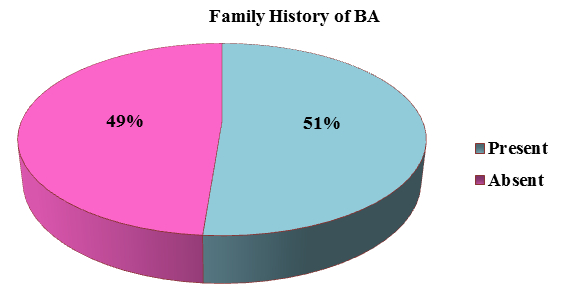
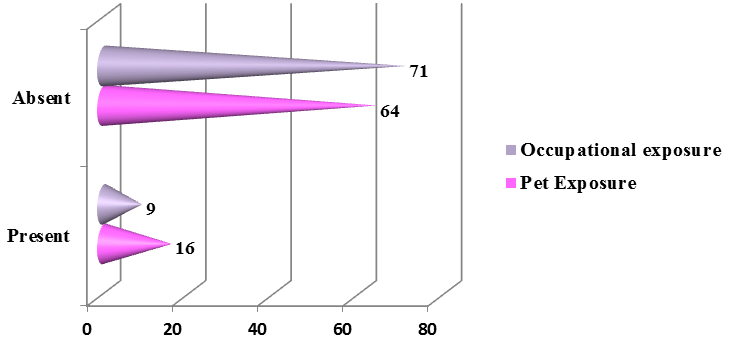
The family history of bronchial asthma was depicted in the Figure 4 in which more than half of the presented patients (51%) had family history suggestive of bronchial asthma. About 11.25 % (9) of the patients had an occupational exposure like chemical fumes, farming, and housekeeping and about 20% (16) of the patients had exposure to dogs predominantly and cats and parrots was depicted in the Figure 5.
Comparison of hsCRP of three groups
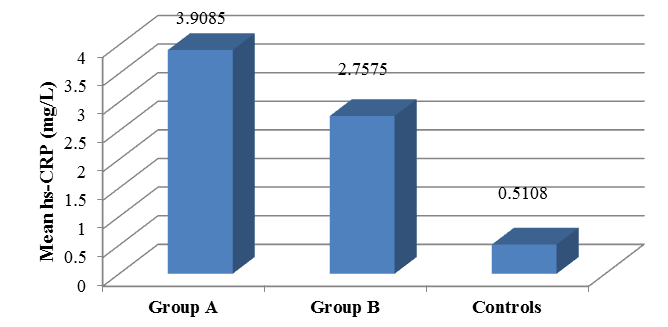
The comparison of hsCRP of three groups Group A (Asthmatic patients who are steroid naïve), Group B (Asthmatic patients who use ICS) and Group C (Normal healthy control). The mean hs CRP of Group A patients total numbers are 40 ± 3.90 in Group B patients 40 ± 2.75 and in control group 24 ± 0.51. The data are plotted in Table 3 , and bar graph diagram Figure 6.
It is being noted that hsCRP levels are increased in both groups of asthmatic patients irrespective of use of steroids and when statistically compared, Group A has a mean value of hsCRP of (3.9085) which was higher than that of group B (2.7575) and that of healthy control group (0.5108). The comparative studies of hsCRP results between Group A and Group B shows that hsCRP elevated in both Group A (asthmatic patients who are steroid naïve or not using ICS) and Group B (asthmatic patients who are using ICS), the difference between the means of hsCRP among the two groups was not statistically significant (p=0.093) with a p value is > 0.05. Further hsCRP comparative studies between asthmatic patients (Group A+B) and healthy controls (Group C) shows that the difference between the means of hsCRP of both groups compared with healthy controls showed high statistical significance (p=0.001).
Correlation of hsCRP with pulmonary function tests
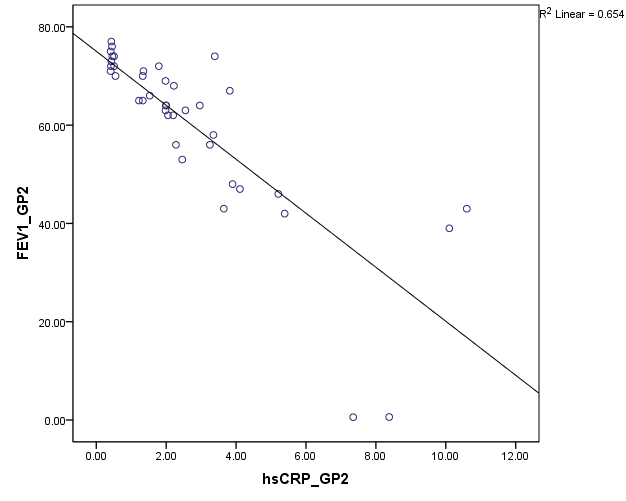
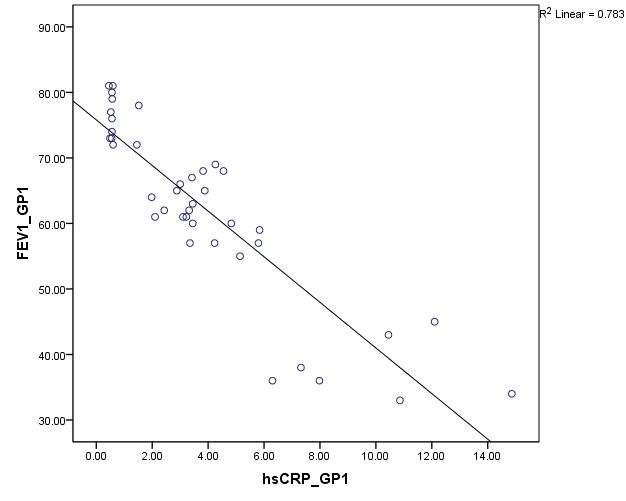
Pulmonary function test using Spirometer was s done in all the patients (group A and B). The FEV1 values are compared against hsCRP values. Statistical significance is assessed using Spearmann’s correlation. From Figure 7, among group B, hsCRP has a significant negative correlation with FEV1 and FEV1/ FVC. Group A patients showing high significant negative correlation of hsCRP with FEV1 and FEV1/FVC (Fig:8).
Table 1
Gender distribution comparison between group A and B
| Steroid naïve (Group A) | Patients using ICS (Group B) | Normal control (Group C) | ||||
| Gender | Count | % | Count | % | Count | % |
| Male | 21 | 52.5 | 17 | 42.5 | 14 | 58.3 |
| Female | 19 | 47.5 | 23 | 57.5 | 10 | 41.6 |
| Total | 40 | 100 | 40 | 100 | 24 | |
Table 2
Age distribution compared between two groups
Table 3
Comparison of hsCRP of three groups
| hs-CRP | N | Mean | Std. Deviation |
| Group A | 40 | 3.9085 | 3.43739 |
| Group B | 40 | 2.7575 | 2.55581 |
| Control | 24 | 0.5108 | 0.4127 |
Discussion
Several studies have reported elevation of the markers of systemic inflammation, such as high-sensitivity C-reactive protein (hs-CRP) in the blood of COPD patients when compared without COPD. CRP is a plasma protein which belongs to the pentraxin group, has been consistently used as a marker of inflammation, infection and tissue damage. It is this context the CRP demonstrates as a promising marker to estimate the condition of patients with poorly controlled asthma, brittle asthma and those who are prone to exacerbations and episodes of acute severe asthma.
Various studies have been conducted in relationship of hsCRP with bronchial asthma patients with control group, they found that hsCRP was increased in asthmatic patients both steroid naïve and steroid inhaling groups when compared to controls with increased bronchial hyper responsiveness of high CRP level. 13, 14 Our study with 80 patients is in concordance with studies done earlirer authors15, 16.
Various studies conducted for the hsCRP in steroid naïve patients Vs steroid users resuls and its analysis proved that significant increase in hsCRP levels in steroid naïve patients when compared to control groups 17, 18. Our study showed no significant correlation (p=0.093) of hsCRP in patients using inhaled steroids and patients not using ICS , which is in concordance with earlier authers 13, 14. hsCRP and pulmonary function tests documented by earier authors proved that asthmatic patients with high hs CRP have more severe obstruction with low FEV1values and also with high hs CRP levels showed significant increase in respiratory symptoms of wheeze, attacks of breathlessness and nocturnal cough it can be concluded hsCRP as sensitive marker for severe asthma 19 .
There are some limitation in our study which inclues, less number of patients and controls. If there are more sample size a clear and concrete conclusion can be drawn. And some of the patients diagnosed are probably on systemic corticosteroids to control their shortness of breath.
Conclusions
However our study results demonstrated that, the hsCRP is a sensitive, easily measured inflammatory marker and is a useful systemic biomarker of airway inflammation in asthmatic patients. Increased levels of hsCRP in asthmatic patients irrespective of ICS treatment and its correlation to pulmonary function reflect the fact that asthma is not only characterised by local airway inflammation but also has a component of low grade systemic inflammation. Patients with decreased pulmonary function showed an association with higher serum hsCRP level more in steroid naive patients. Therefore, serum hsCRP can be used as one of the indirect parameters to detect the degree of severity affecting the airways.